Abstract
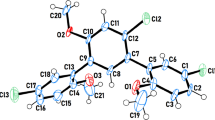
Two fluorine and bromine Boc protected phenoxypropanamine compounds were investigated to determine how hydrogen bonds affect the dynamic behaviour of an organic molecule. Using single crystal X-ray diffraction, the presence of a folded backbone for the fluorine-containing compound was established. In addition, the presence of an inter-molecular (conventional and non-conventional) hydrogen bond was detected by XRD. To investigate the conformational preference and available interactions in solution, variable temperature NMR at two different concentrations, 2D NMR at low temperature and quantum mechanics studies were performed. These investigations confirmed that the observed interactions in the solid phase were maintained in solution. However, competition between inter- and intra non-conventional hydrogen bond (originating from fluorine atoms) was detected at high temperature in dilute solution. Our studies suggest that fluorine assisted inter-molecular cross-coupling at room temperature. This study possibly introduces a new tool for manipulation of peptide and protein structures towards desired biological applications.
Graphical abstract








Similar content being viewed by others
References
Albericio F, Kruger HG (2012) Future Med Chem 4:1527
Pattabiraman VR, Bode JW (2011) Nature 480:471
Johansson A, Kollman P, Rothenberg S, McKelvey J (1974) J Am Chem Soc 96:3794
Kollman PA, Allen LC (1972) Chem Rev 72:283
Desiraju GR (1996) Acc Chem Res 29:441
Laursen JS, Engel-Andreasen J, Fristrup P, Harris P, Olsen CA (2013) J Am Chem Soc 135:2835
Ward MD, Raithby PR (2013) Chem Soc Rev 42:1619
Steiner T (2002) Angew Chem Int Ed 41:49
Emenike BU, Carroll WR, Roberts JD (2013) J Org Chem 78:2005
Nagy PI (2013) J Phys Chem A 117:2812
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Acc Chem Res 38:386
Fuller RO, Griffith CS, Koutsantonis GA, Lapere KM, Skelton BW, Spackman MA, White AH, Wild DA (2012) CrystEngComm 14:804
Hunter CA (2004) Angew Chem Int Ed 43:5310
Mati IK, Cockroft SL (2010) Chem Soc Rev 39:4195
Scharf DH, Chankhamjon P, Scherlach K, Heinekamp T, Willing K, Brakhage AA, Hertweck C (2013) Angew Chem Int Ed 52:11092
Yang L, Adam C, Nichol GS, Cockroft SL (2013) Nat Chem 5:1006
Chiarucci M, Ciogli A, Mancinelli M, Ranieri S, Mazzanti A (2014) Angew Chem Int Ed 53:5405
Parthasarathi R, Subramanian V (2006) Characterization of hydrogen bonding: from van der Waals interactions to covalency. In: Grabowski SJ (ed) Hydrogen bonding—new insights. Springer, Dordrecht
Reichardt C, Welton T (2011) Solvents and solvent effects in organic chemistry, 4th edn. Wiley, New York
Kohli R (2016) Hydrogen bonding ability of hydroxamic acid and its isosteres with water and amino acid side chain groups, 1st edn. Anchor Academic Publishing, Germany
Taylor R (2016) Cryst Growth Des 16:4165
Alkorta I, Elguero J (1998) Chem Soc Rev 27:163
Jones CR, Qureshi MKN, Truscott FR, Hsu S-TD, Morrison AJ, Smith MD (2008) Angew Chem Int Ed 47:7099
Kovács A, Varga Z (2006) Coord Chem Rev 250:710
Preimesberger MR, Majumdar A, Aksel T, Sforza K, Lectka T, Barrick D, Lecomte JTJ (2015) J Am Chem Soc 137:1008
Desiraju GR (2011) Angew Chem Int Ed 50:52
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P, Kjaergaard HG, Legon AC, Mennucci B, Nesbitt DJ (2011) Pure Appl Chem 83:1637
Perrin CL, Burke KD (2014) J Am Chem Soc 136:4355
Lakshmipriya A, Suryaprakash N (2016) J Phys Chem A 120:7810
Lakshmipriya A, Chaudhari SR, Shahi A, Arunan E, Suryaprakash N (2015) Phys Chem Chem Phys 17:7528
Mishra SK, Suryaprakash N (2015) RSC Adv 5:86013
Alapour S, Ramjugernath D, Koorbanally NA (2015) RSC Adv 5:83576
Desiraju GR, Steiner T (2001) The weak hydrogen bond: in structural chemistry and biology, 1st edn. Oxford University Press, New York
Bellisent-Funel MC, Dore JC (2013) Hydrogen bond networks, 1st edn. Springer, Netherlands
Farahani MD, Honarparvar B, Albericio F, Maguire GEM, Govender T, Arvidsson PI, Kruger HG (2014) Org Biomol Chem 12:4479
Desiraju GR (2002) Acc Chem Res 35:565
Abraham R, Mobli M (2008) Modelling 1H NMR spectra of organic compounds: theory, applications and NMR prediction software, 1st edn. Wiley, London
Chaudhari SR, Mogurampelly S, Suryaprakash N (2013) J Phys Chem B 117:1123
Bower JF, Szeto P, Gallagher T (2007) Org Lett 9:3283
Chen W, Twum EB, Li L, Wright BD, Rinaldi PL, Pang Y (2012) J Org Chem 77:285
Clayden J, Greeves N, Warren S (2012) Organic chemistry, 2nd edn. OUP, Oxford
Aihara J-I (1999) J Phys Chem A 103:7487
Alves CN, Carneiro AS, Andrés J, Domingo LR (2006) Tetrahedron 62:5502
Borges RS, Vale JKL, Pereira GAN, Veiga AAS, Junior JB, da Silva ABF (2016) Med Chem Res 25:852
Borges RS, Barros TG, Veiga AAS, Carneiro AS, Barros CAL, da Silva ABF (2015) Med Chem Res 24:3453
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford
Mennucci B (2012) Wiley Interdiscip Rev Comput Mol Sci 2:386
Acknowledgements
This research was supported by grants from the National Research Foundation (NRF) South Africa and the South African Research Chairs Initiative of the Department of Science and Technology. We thank Mr. Dilip Jagjivan for his assistance with NMR experiments. JRAS and CAN thank CAPES and CNPq Brazilian Agencies and CHPC (http://www.chpc.ac.za) for computational resources.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alapour, S., Farahani, M.D., Silva, J.R.A. et al. Investigation of conventional and non-conventional hydrogen bonds: a comparison of fluorine-substituted and non-fluorine substituted compounds. Monatsh Chem 148, 2061–2068 (2017). https://doi.org/10.1007/s00706-017-2044-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2044-3