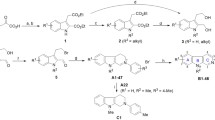
Abstract
This present paper describes the preparation and characterization of a series of O-substituted N-cycloalkylcarbamate derivatives. These compounds were tested as inhibitors of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). All studied carbamates exhibited moderate inhibitory activity of both cholinesterases with values of IC50 in the range of 36.1–78.6 μM for AChE and 9.8–215.4 μM for BChE, respectively. These values are comparable with those values of inhibition obtained with the established drug rivastigmine. The cytotoxicity of all carbamates was evaluated using standard in vitro test with Jurkat cells. Many of the studied carbamates can be considered as promising compounds for potential medicinal applications with regard to their inhibitory activity as well as negligible cytotoxicity.
Graphical abstract


Similar content being viewed by others
References
Alzheimer’s Association (2014) Alzheimer’s Association report: 2014 Alzheimer’s disease facts and figures. Alzheimers Dement 10:e47
Lu LC, Bludau JH (2001) Alzheimer’s disease. Greenwood Publishing Group, Santa Barbara
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Lancet 377:1019
Amemori T, Jendelova P, Ruzicka J, Urdzikova LM, Sykova E (2015) Int J Mol Sci 16:26417
Alzheimer’s Association (2015) Alzheimer’s Association report: 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11:332
Contestabile A (2011) Behav Brain Res 221:334
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) J Neurol Neurosurg Psychiatry 66:137
Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM (2013) Curr Neuropharmacol 11:315
Khan MA, Fazal-ur-Rehman S, Hameed A, Kousar S, Dalvandi K, Yousuf S, Choudhary MI, Basha FZ (2015) RSC Adv 5:59240
Toda N, Tago K, Marumoto S, Takami K, Ori M, Yamada N, Koyama K, Naruto S, Abe K, Yamazaki R, Hara T, Aoyagi A, Abe Y, Kaneko T, Kogen H (2003) Bioorg Med Chem 11:1935
Zheng X-Y, Zhang Z-J, Chou G-X, Wu T, Cheng X-M, Wang C-H, Wang Z-T (2009) Arch Pharmacol Res 32:1245
Savini L, Campiani G, Gaeta A, Pellerano C, Fattorusso C, Chiasserini L, Fedorko JM, Saxena A (2001) Bioorg Med Chem Lett 11:1779
Bacalhau P, San Juan AA, Goth A, Caldeira AT, Martins R, Burke AJ (2016) Bioorg Chem 67:105
Sawatzky E, Wehle S, Kling B, Wendrich J, Bringmann G, Sotriffer CA, Heilmann J, Decker M (2016) J Med Chem 59:2067
Liew K-F, Chan K-L, Lee C-Y (2015) Eur J Med Chem 94:195
Bohn P, Gourand F, Papamicaël C, Ibazizène M, Dhilly M, Gembus V, Alix F, Tintas M-L, Marsais F, Barré L, Levacher V (2015) ACS Chem Neurosci 6:737
Sedlák M, Hanusek J, Drabina P, Štěpánková Š, Čegan A (2009) Arkivoc vii:1
Imramovský A, Pejchal V, Štěpánková Š, Vorčáková K, Jampílek J, Vančo J, Šimůnek P, Královec K, Brůčková L, Mandíková J, Trejtnar F (2013) Bioorg Med Chem 21:1735
Vorčáková K, Štěpánková Š, Sedlák M, Vytřas K (2015) Chemosensors 3:274
Saxena J, Meloni D, Huang M-T, Heck DE, Laskin JD, Heindel ND, Young SC (2015) Bioorg Med Chem Lett 25:5609
Camerino E, Wong DM, Tong F, Körber F, Gross AD, Islam R, Viayna E, Mutunga JM, Li J, Totrov MM, Bloomquist JR, Carlier PR (2015) Bioorg Med Chem Lett 25:4405
Verma A, Wong DM, Islam R, Tong F, Ghavami M, Mutunga JM, Slebodnick C, Li J, Viayna E, Lam PC-H, Totrov MM, Bloomquist JR, Carlier PR (2015) Bioorg Med Chem 23:1321
Anand P, Singh B (2013) Med Chem 9:694
Anand P, Singh B (2013) Med Chem Res 22:1648
Ma H-J, Xie R-L, Zhao Q-F, Mei X-D, Ning J (2010) J Agric Food Chem 58:12817
Pohanka M (2011) Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155:219
Heinig R, Zimmer D, Yeh S, Krol GJ (2000) J Chromatogr B 741:257
Ibach B, Haen E (2004) Curr Pharm Des 10:231
Horáková E, Drabina P, Brož B, Štěpánková Š, Vorčáková K, Královec K, Havelek R, Sedlák M (2016) J Enzym Inhib Med Chem 31:173
Hardegger LA, Kuhn B, Spinnler B, Anselm L, Ecabert R, Stihle M, Gsell B, Thoma R, Diez J, Benz J, Plancher J-M, Hartmann G, Banner DW, Haap W, Diederich F (2011) Angew Chem Int Ed 50:314
Riley KE, Hobza P (2011) Cryst Growth Des 11:4272
Hardegger LA, Kuhn B, Spinnler B, Anselm L, Ecabert R, Stihle M, Gsell B, Thoma R, Diez J, Benz J, Plancher J-M, Hartmann G, Isshiki Y, Morikami K, Shimma N, Haap W, Banner DW, Diederich F (2011) ChemMedChem 6:2048
Zhou P, Huang J, Tian F (2012) Curr Med Chem 19:226
Lu YX, Liu YT, Xu ZJ, Li HY, Liu HL, Zhu WL (2012) Expert Opin Drug Discov 7:375
Wilcken R, Zimmermann MO, Lange A, Zahn S, Boeckler FM (2012) J Comput Aided Mol Des 26:935
Scholfield MR, Vander Zanden CM, Carter M, Ho PS (2013) Protein Sci 22:139
Wilcken R, Zimmermann MO, Lange A, Joerger AC, Boeckler FM (2013) J Med Chem 56:1363
Sirimulla S, Bailey JB, Vegesna R, Narayan M (2013) J Chem Inf Model 53:2781
Xu ZJ, Yang Z, Liu YT, Lu YX, Chen KX, Zhu WL (2014) J Chem Inf Model 54:69
Ho PS (2015) Biomolecular halogen bonds. In: Metrangolo P, Resnati G (eds) Halogen bonding I: impact on materials chemistry and life sciences. Topic in current chemistry. Springer, Berlin
Persch E, Dumele O, Diederich F (2015) Angew Chem Int Ed 54:3290
Yang Z, Liu YT, Chen ZQ, Xu ZJ, Shi JY, Chen KX, Zhu WL (2015) J Mol Model 21:138
Cavallo G, Metrangolo P, Milani R, Pilati T, Priimagi A, Resnati G, Terraneo G (2016) Chem Rev 116:2478
Jiang SQ, Zhang LJ, Cui DB, Yao ZQ, Gao B, Lin JP, Wei DZ (2016) Sci Rep 6:34750
Evans DA, Woerpel KA, Hinman MM, Faul MM (1991) J Am Chem Soc 113:726
Chen Y, Zhang XP (2007) J Org Chem 72:5931
Concellón JM, Rodruguez-Solla H, Simal C (2007) Org Lett 9:2685
Gee YS, Goertz NJM, Gardiner MG, Hyland CJT (2016) Org Biomol Chem 14:2498
Christiansen E, Urban C, Grundmann M, Due-Hansen ME, Hagesaether E, Schmidt J, Pardo L, Ullrich S, Kostenis E, Kassack M, Ulven T (2011) J Med Chem 54:6691
Rappopot Z (ed) (1987) The chemistry of the cyclopropyl group, vol 1–2. Wiley-VCH, New York
Tedeschi RE (1961) Monoamine oxidase inhibition. US Patent 2,997,422, 22 Aug 1961
Tedeschi RE (1962) Chem Abstr 56:7554
Bridges AJ, Sanchez JP (1990) J Heterocycl Chem 27:1527
Chernykh AV, Radchenko DS, Chernykh AV, Kondratov IS, Tolmachova NA, Datsenko OP, Kurkunov MA, Zozulya SX, Kheylik YP, Bartels K, Daniliuc CG, Haufe G (2015) Eur J Org Chem 2015:6466
Burgess LE (2009) 6-Substituted phenoxychroman carboxylic acid derivatives. WO Patent WO 2009158426 A1, 30 Dec 2009
Burgess LE (2009) Chem Abstr 152:119424
Leo A, Hansch C, Elkins D (1971) Chem Rev 71:525
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Adv Drug Deliv Rev 46:3
Lipinski CA (2004) Drug Discov Today Technol 1:337
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) Biochem Pharmacol 7:88
Krátký M, Štěpánková Š, Vorčáková K, Navrátilová L, Trejtnar F, Stolaříková J, Vinšová J (2017) Bioorg Chem 71:244
Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, Yu QS, Mamczarz J, Holloway HW, Giordano T, Chen D, Furukawa K, Sambamurti K, Brossi A, Lahiri DK (2005) Proc Natl Acad Sci 102:17213
Giacobini E (2004) Pharmacol Res 50:433
Brus B, Košak U, Turk S, Pišlar A, Coquelle N, Kos J, Stojan J, Colletier JP, Gobec S (2014) J Med Chem 57:8167
Benelkebir H, Hodgkinson C, Duriez PJ, Hayden AL, Bulleid RA, Crabb SJ, Packham G, Ganesan A (2011) Bioorg Med Chem 19:3709
Wurz RP, Charette AB (2004) J Org Chem 69:1262
Vianello P, Botrugno OA, Cappa A, Dal Zuffo R, Dessanti P, Mai A, Marrocco B, Mattevi A, Meroni G, Minucci S, Stazi G, Thaler F, Trifiro P, Valente S, Villa M, Varasi M, Mercurio C (2016) J Med Chem 59:1501
Woodroofe CC, Zhong B, Lu X, Silverman RB (2000) J Chem Soc Perkin Trans 2:55
Tsui GC, Menard F, Lautens M (2010) Org Lett 12:2456
Sangster J (1989) Phys Chem Ref Data 18:1111
OECD Guidelines for the testing of chemicals, section 1, books/OECD guidelines for the testing of chemicals, section 1/test no. 107: partition coefficient (n-octanol/water): shake flask method. http://www.oecd-ilibrary.org/environment/test-no-107-partition-coefficient-n-octanol-water-shake-flask-method_9789264069626-en. Accessed 20 Feb 2017
Havelek R, Siman P, Cmielova J, Stoklasova A, Vavrova J, Vinklarek J, Knizek J, Rezacova M (2012) Med Chem 8:615
Acknowledgements
The authors acknowledge the financial support of the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Jaromír Kaválek on the occasion of his 80th birthday.
Rights and permissions
About this article
Cite this article
Horáková, E., Drabina, P., Brůčková, L. et al. Synthesis and in vitro evaluation of novel N-cycloalkylcarbamates as potential cholinesterase inhibitors. Monatsh Chem 148, 2143–2153 (2017). https://doi.org/10.1007/s00706-017-2026-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2026-5