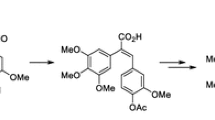
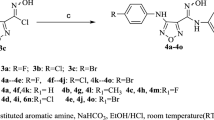
Abstract
A series of benzo[b]furans was synthesized with modification at the 5-position of the benzene ring by introducing different aryl acetylenyl and acrylic acid moiety as combretastatin A-4 analogues. The compounds were evaluated by MTT assay for cytotoxic effects against lung cancer (A549), liver cancer (BEL-7404), colon cancer (SW620), and cervical cancer (HeLa) cell lines and the compounds were more sensitive to A549 and Hela cell lines. One compound exhibited the highest inhibition against A549 and Hela cells (IC50 = 0.18 and 0.14 μM) and very close to that of CA-4 (IC50 = 0.16 and 0.12 μM). This compound elicited cell-cycle arrest in the G2/M phase and significantly induced apoptosis in HeLa cells. Molecular docking studies indicated that it displayed hydrogen-bonding interactions with the amino acid residues Gln-11 and Tyr-224 would be a potential tululin inhibitor.
Graphical abstract








Similar content being viewed by others
References
Pettit GR, Cragg GM, Herald DL, Schmidt JM (1982) Can J Chem 60:1374
Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D (1989) Experientia 45:209
Chaplin DJ, Hill SA (2002) Int J Radiat Oncol Biol Phys 54:1491
Pettit GR, Temple C Jr, Narayanan VL, Varma R, Simpson MJ, Boyd MR, Rener GA, Bansal N (1995) Anti-Cancer Drug Des 10:299
Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA (2006) J Med Chem 49:3033
Pettit GR, Minardi MD, Rosenberg HJ, Hamel E, Bibby MC, Martin SW, Jung MK, Pettit RK, Cuthbertson TJ, Chapuis JC (2005) J Nat Prod 68:1450
Fortin S, Moreau E, Lacroix J, Teulade JCC, Patenaude A, Gaudreault C-R (2007) Bioorg Med Chem Lett 17:2000
Simoni D, Romagnoli R, Baruchello R, Rondanin R, Grisolia G, Eleopra M, Rizzi M, Tolomeo M, Giannini G, Aloatti D, Castorina M, Marcellini M, Pisano C (2008) J Med Chem 51:6211
Dzierzbicka K, Kubacka P, Renusz S, Kołodziejczyk A (2008) Wiad Chem 62:1037
Chaudhary A, Pandeya SN, Kumar P, Sharma PP, Gupta S, Soni N, Verma KK, Bhardwaj G (2007) Mini Rev Med Chem 7:1186
Jaroch K, Karolak M, Górski P, Jaroch A, Krajewski A, Ilnicka A, Sloderbach A, Stefanski T, Sobiak S (2016) Pharmacol Rep 68:1266
Vonreuβ SH, König WA (2004) Phytochemistry 65:3113
Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Olga C-L, Tolomeo M, Grimaudo S, Cristina AD, Pipitone MR, Balzarini J, Zonta N, Brancale A, Hamel E (2009) Bioorg Med Chem 17:6862
Kamal A, Reddy NVS, Nayak VL, Reddy VS, Prasad B, Nimbarte VD, Srinivasulu V, Vishnuvardhan MVPS, Reddy CS (2014) Chem Med Chem 9:117
Kamal A, Nayak VL, Nagesh N, Vishnuvardhan MVPS, Reddy NVS (2016) Bioorg Chem 66:124
Rao MLN, Awasthi DK, Banerjee D (2007) Tetrahedron Lett 48:431
Thorand S, Krause N (1998) J Org Chem 63:8551
Massue J, Frath D, Retailleau P, Ulrich G, Ziessel R (2013) Chem Eur J 19:5375
Sylvie D, David R, Meiko W, Alexander K, Jérémie FDC, Alan TM, Nicholas JL (2009) Bioorg Med Chem 17:7698
Fan L, Weimin Y, Dongsheng G, Zhiquan H, Hua X, Zhangqun Y (2011) Oncol Rep 25:1629
Bai L, Wang R, Zuo Y, Xu G (2015) Chem Res Chin Univ 31:964
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew R K, Goodsell DS, Olson AJ (2009) J Comput Chem 30:2785; http://autodock.scripps.edu
PyMOL Molecular Graphics System, version 1.7.4 (2015) Schrödinger, LLC, New York; http://www.pymol.org
Acknowledgements
This research was funded by Scientific Research Program of the Higher Education Institution of Xin Jiang (FSRPHEXJ Project No. XJEDU2016I031) and Xinjiang Medical University (Project No. CXCY097). We thank them for the financial support given to the research projects.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, Y., Luo, Y. & Ma, C. Synthesis and cytotoxic evaluation of combretastatin A-4 analogues of benzo[b]furans. Monatsh Chem 148, 1823–1832 (2017). https://doi.org/10.1007/s00706-017-2001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2001-1