Abstract
New phenolic derivatives bearing hydrazine and 1,3,4-oxadiazole moieties were synthesized and evaluated for their in vitro antimicrobial and antioxidant activities. Most of the compounds revealed pronounced activity against Pseudomonas aeruginosa as well as promising antioxidant activities. N 1-(2,5-Dihydroxybenzoyl)-N 2-(4-methylphenylsulfonyl)hydrazine displayed promising activity against Escherichia coli and P. aeruginosa. N 1-(2,5-Dihydroxybenzoyl)-N 2-(2-naphthalenylmethylene)hydrazine was almost equipotent to the standard antioxidant vitamin C having scavenging activities of 84 and 93%, respectively. In vitro cytotoxicity study revealed that N 1-(2,5-dihydroxybenzoyl)-N 2-(2,3,4-trimethoxyphenylmethylene)hydrazine, N 1-(2,5-dihydroxybenzoyl)-N 2-(3,4,5-trimethoxyphenylmethylene)hydrazine, and N 1-(2,5-dihydroxybenzoyl)-N 2-(4-methylphenylsulfonyl)hydrazine are more safe than reference 5-fluorouracil. In silico drug relevant properties proposed that all compounds have high to moderate drug-likeness scores. Accordingly, these derivatives can be potential leads for development of potent antimicrobial and antioxidant agents.
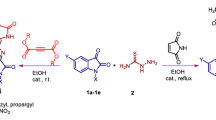
Graphical abstract


Similar content being viewed by others
References
Demain AL, Sanchez S (2009) J Antibiot 62:5
Pisoschi AM, Pop A (2015) Eur J Med Chem 97:55
Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J, Nishigaki I (2014) Clin Chim Acta 436:332
Aruoma O (1998) J Am Oil Chem Soc 75:199
Gutiérrez-Larraínzar M, Rúa J, Caro I, de Castro C, de Arriaga D, García-Armesto MR, del Valle P (2012) Food Control 26:555
Gyawali R, Ibrahim SA (2014) Food Control 46:412
Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E (2008) Food Chem Toxicol 46:3774
Wang W, Guo J, Zhang J, Peng J, Liu T, Xin Z (2015) Food Chem 171:40
Zhang B, Deng Z, Ramdath DD, Tang Y, Chen PX, Liu R, Liu Q, Tsao R (2015) Food Chem 172:862
Sumczynski D, Bubelova Z, Sneyd J, Erb-Weber S, Mlcek J (2015) Food Chem 174:319
Sakihama Y, Cohen MF, Grace SC, Yamasaki H (2002) Toxicology 177:67
Bendich A, Machlin LJ, Scandurra O, Burton GW, Wayner DDM (1986) Adv Free Radic Biol Med 2:419
Craft BD, Kerrihard AL, Amarowicz R, Pegg RB (2012) Compr Rev Food Sci Food Saf 11:148
Nihei K-I, Nihei A, Kubo I (2003) Bioorg Med Chem Lett 13:3993
Balan AM, Florea O, Moldoveanu C, Zbancioc G, Iurea D, Mangalagiu II (2009) Eur J Med Chem 44:2275
Georgiev L, Chochkova M, Totseva I, Seizova K, Marinova E, Ivanova G, Ninova M, Najdenski H, Milkova T (2013) Med Chem Res 22:4173
Barontini M, Bernini R, Carastro I, Gentili P, Romani A (2014) New J Chem 38:809
Kajiyama T, Ohkatsu Y (2001) Polym Degrad Stab 71:445
Li Q, Chen J, Luo S, Xu J, Huang Q, Liu T (2015) Eur J Med Chem 93:461
Shenvi S, Kumar K, Hatti KS, Rijesh K, Diwakar L, Reddy GC (2013) Eur J Med Chem 62:435
Zuo A, Yanying Y, Li J, Binbin X, Xiongying Y, Yan Q, Shuwen C (2011) Free Radic Antioxid 1:39
Chaaban I, El Khawass EM, Abd El Razik HA, El Salamouni NS, Redondo-Horcajo M, Barasoain I, Díaz JF, Yli-Kauhaluoma J, Moreira VM (2016) Arch Pharm 349:1
Chaaban I, El Khawass EM, Abd El Razik HA, El Salamouni NS, Redondo-Horcajo M, Barasoain I, Díaz JF, Yli-Kauhaluoma J, Moreira VM (2014) Eur J Med Chem 87:805
Musad EA, Mohamed R, Ali Saeed B, Vishwanath BS, Rai KM (2011) Bioorg Med Chem Lett 21:3536
Shyma PC, Kalluraya B, Peethambar SK, Telkar S, Arulmoli T (2013) Eur J Med Chem 68:394
Arunkumar S, Ilango K, Manikandan RS, Sudha M, Ramalakshmi N (2009) Int J Chem Tech 1:1094
Guimarães CR, Boger DL, Jorgensen WL (2005) J Am Chem Soc 127:17377
Pieczonka AM, Strzelczyk A, Sadowska B, Mlostoń G, Stączek P (2013) Eur J Med Chem 64:389
Morjan RY, Mkadmh AM, Beadham I, Elmanama AA, Mattar MR, Raftery J, Pritchard RG, Awadallah AM, Gardiner JM (2014) Bioorg Med Chem Lett 24:5796
Renuka N, Kumar KA (2013) Bioorg Med Chem Lett 23:6406
Kagthara PR, Shah NS, Doshi RK, Parekh HH (1999) Indian J Chem 38B:572
Siddique M, Saeed AB, Ahmad S, Dogar NA (2013) J Sci Innov Res 2:628
Vanucci-Bacqué C, Carayon C, Bernis C, Camare C, Nègre-Salvayre A, Bedos-Belval F, Baltas M (2014) Bioorg Med Chem 22:4269
Padmaja A, Pedamalakondaiah D, Sravya G, Reddy GM, Kumar MVJ (2015) Med Chem Res 24:2011
Roussaki M, Zelianaios K, Kavetsou E, Hamilakis S, Hadjipavlou-Litina D, Kontogiorgis C, Liargkova T, Detsi A (2014) Bioorg Med Chem 22:6586
Chaaban I, El Khawass EM, Mahran MA, Abd El Razik HA, El Salamouni NS, Abdel Wahab AE (2013) Med Chem Res 22:3760
Chaaban I, El Khawass EM, Mahran MA, Abd El Razik HA, El Salamouni NS, Abdel Wahab AE (2013) Med Chem Res 22:841
Fox HH, Gibas JT (1952) J Org Chem 17:1653
Blois MS (1958) Nature 181:1199
Rahman A, Shahabuddin G, Hadi SM, Parish JH, Ainley K (1989) Carcinogenesis 10:1833
Papuc C, Predescu NC, Nicorescu V (2013) Bull UASVM Vet Med 70:162
Desmarchelier C, Coussio J, Ciccia G (1998) Braz J Med Biol Res 31:1163
http://www.organicchemistry.org/prog/peo/. Accessed 16 Nov 2016
http://www.chemaxon.com/products/marvin/marvin-sketch/. Accessed 16 Nov 2016
Chaaban I (1980) Sci Pharm 48:356
Jain SR, Kar A (1971) Planta Med 20:118
Scott AC (1989) In: Collee JG, Duguid JP, Fraser AG, Marmion BP (eds) Mackie & McCartney practical medical microbiology, vol 2, 13th edn. Churchill Livingstone, Edinburgh, pp 161–181
Böyum A (1968) Scand J Clin Lab Investig Suppl 97:77
Borenfreund E, Puerner JA (1984) J Tissue Cult Meth 9:7
GraphPad Software, San Diego, California, USA. http://www.graphpad.com. Accessed 1 Oct 2016
Acknowledgements
Authors thank the University of Helsinki, Finland for doing part of the chemistry work, NMR and IR spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaaban, I., El Khawass, E.S.M., Abd El Razik, H.A. et al. Synthesis and evaluation of new phenolic derivatives as antimicrobial and antioxidant agents. Monatsh Chem 149, 127–139 (2018). https://doi.org/10.1007/s00706-017-1983-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-1983-z