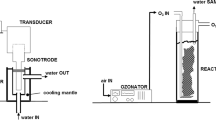
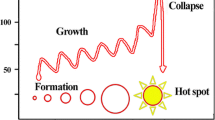
Abstract
The necessity of leachates treatment from the waste landfills by new ways becomes increasingly more marked. Leachates contain toxic ingredients such as pesticides, dyes, and hormonal agents, which necessarily need to be removed from the environment. It is because of their genotoxicity and estrogenic activity. The main aim of this article is to point out the possibility of using ultrasound in removing ammonia from hazardous landfills leachate. In addition to ultrasound degradation, the possibility of addition of the H2O2 was also studied. System ultrasound/H2O2 was proved to be more effective in removing ammonia and organic pollution than ultrasound alone. Tested leachate contains 3.20 g/dm3 N-NH4 +, 15 mg/dm3 CN−, and 4.4 g/dm3 chemical oxygen demand (COD). We achieved degradation efficiency of 63% of ammonia which means final concentration of 1.12 g/dm3 N-NH4 +, after using pretreatment with the most efficient system H2O2 and ultrasound. COD removal efficiency was 56% (1.95 g/dm3 COD). We achieved the value 10.2 mg/dm3 for cyanide, which means 65%.
Graphical abstract





Similar content being viewed by others
References
Pitter P (2007) Hydrochemie. VŠCHT, Praha
Dohányos M, Koller J, Strnadová N (1998) Čištění odpadních vod. VŠCHT, Praha
Mackuľak T, Smolinská M, Takáčová A, Bodík I, Škubák J, Kunštek M (2013) Vodní hospodářství 2:51
Rivas FJ, Beltrán F, Gimeno O, Carvalho F (2003) J Environ Sci Heal A 38:371
Yoon J, Cho S, Cho Y, Kim S (1998) Water Sci Technol 38:209
Necyaj E, Okoniewska E, Kacprzak M (2005) Desalination 182:357
Singh R, Bishnoi RN, Kirrolia A, Kumar R (2013) Bioresour Technol 127:49
Čerňanský S (2014) Biologické remediácie. Vysoká škola báňská, Technická univerzita Ostrava, Ostrava
Chang JS, Law R, Chang C (1997) Water Res 31:1651
Liu HL, Chen BY, Lan YW, Cheng YC (2004) Chem Eng J 97:195
Fairbrother L, Shapter J, Brugger J, Southam G, Pring A, Reith F (2009) Chem Geol 265:313
García DN, Gómez ZA, Navalón A, González J, Vílchez LJ (2013) Sci Total Environ 442:317
Moravia GW, Amaral SCM, Lange CL (2013) Waste Manage 33:89
Prousek J, Priesolová S (2002) Chem Listy 96:893
Prousek J (2007) Pure Appl Chem 79:2325
Brock M, Eggert T, Palisaar JR, Roghmann F, Braun K, Loppenberg B, Sommerer F, Noldus J, Bodman C (2013) J Urol 189:93
Sonn AG, Natarajan S, Margolins JA, MacAiran M, Lieu P, Huang J, Dorey FJ, Marks SL (2013) J Urol 189:86
Arellano PAC, Martínez SS (2007) Int J Hydrogen Energ 32:3163
Yazici YE, Deveci H, Alp I, Uslu T (2007) Desalination 216:209
Li W, Zhou Q, Hua T (2010) Int J Chem Eng 2010:2
Li Y, Hsieh PW, Mahmudov R, Wei X, Huang PC (2013) J Hazard Mater 244–245:403
Štengl V, Šubrt J (2004) Chem Listy 98:324
Ma YH (2012) Sustain Environ Res 22:271
Huang H, Zhang P, Xiao J, Xiao D, Gao F (2016) Ultrason Sonochem. doi:10.1016/j.ultsonch.2016.08.019
Babu GS, Ashokkumar M, Neppolian B (2016) Top Curr Chem 75:374
De La Rochebrochard S, Naffrechoux E, Drogui P, Mercier G, Blais FJ (2013) Ultrason Sonochem 20:109
Foo KY, Hameed BH (2009) J Hazard Mater 171:57
Kurniawan TA, Lo W-H, Chan GYS (2006) Chem Eng J 125:45
Deng Y, Englehardt JD (2007) Waste Manage 27:385
Murray CA, Parsons SA (2004) Water Sci Technol 4:115
Li W, Zhou Q, Hua T (2010) Int J Chem Eng 2010:8
Tizaoui C, Bouselmi L, Mansouri L, Ghrabi A (2007) J Hazard Mater 140:320
Iordache I, Nechita MT, Aelenei N, Rosca I, Apostolescu G, Peptanariu P (2003) Pol J Environ Stud 12:735
Mackuľak T, Prousek J, Švorc Ľ, Ryba J, Škubák J, Drtil M (2013) Desalin Water Treat 51:22
Horáková M (2003) Analytika vody. VŠCHT, Praha
Acknowledgements
This research was conducted with support of the Slovak research and development agency under Contract No. APVV 037212 and the Science Grant Agency of the Slovak Ministry of Education (VEGA) Project Nos. 1/0631/15 and 1/0543/15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tichý, J., Takáčová, A., Smolinská, M. et al. Treatment of hazardous leachate from landfill using ultrasound/H2O2 system. Monatsh Chem 148, 563–570 (2017). https://doi.org/10.1007/s00706-017-1919-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-1919-7