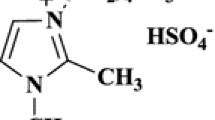
Abstract
Regioselective synthesis of bis-ferrocenylpyrazole derivatives in biphasic aquatic–organic system under catalysis with HBF4 was carried out. The regioselectivity of these reactions depending on the electronic effects of substituents in pyrazole moiety was studied.
Graphical abstract




Similar content being viewed by others
References
Meunier P, Quattara I, Gautheron B, Tirouflet J, Camboli D, Besanson J, Boulay F (1991) Eur J Med Chem 26:351
Popova LV, Babin VN, Belousov YuA, Nekrasov YuS, Snegireva AE, Borodina NP, Shaposhnikova GM, Bychenko OB, Raevskii PM, Morozova NB, Ilyina AI, Shitkov KG (1993) Appl Organomet Chem 7:85
Snegur LV, Simenel AA, Nekrasov YuS, Morozova EA, Starikova ZA, Peregudova SM, Kuzmenko YuV, Babin VN, Ostrovskaya LA, Bluchterova NV, Fomina MM (2004) J Organomet Chem 689:2473
Snegur LV, Nekrasov YuS, Sergeeva NS, Zhilina ZhV, Gumenyuk VV, Starikova ZA, Simenel AA, Morozova NB, Sviridova IK, Babin VN (2008) Appl Organomet Chem 22:139
Simenel AA, Samarina SV, Snegur LV, Starikova ZA, Ostrovskaya LA, Bluchterova NV, Fomina MM (2008) Appl Organomet Chem 22:276
Yaschenko GN, Shashmurina AA, Gorelova GM, Evstigneeva NG, Alekseeva LV, Radina LB (1978) Pharm Chem J 12:68
Snegur LV, Simenel AA, Nekrasov YS, Morozova EA, Starikova ZA, Peregudova SM, Kuzmenko YuV, Babin VN, Ostrovskaya LA, Bluchterova NV, Fomina MM (2004) J Organomet Chem 68:2473
Oparin DA, Makhaev VD, Vilchevskaya VD, Zimatkina TI, Motylevich ZhV, Zimatkin SM, Zabrodskaya SV, Krylova AI, Gorelikova Yu (1996) Pharm Chem J (Engl Transl) 30:79 ((1996) Khim Farm Zh 30:11)
Köpf-Maier P, Köpf H (1987) Chem Rev 87:1137
Köpf-Maier P (1994) Eur J Clinic Pharm 47:1
Babin VN, Raevskii PM, Shitkov KG, Snegur LV, Nekrasov YuS (1995) Mendeleev Chem J 39:17
van Staveren DR, Metzler-Nolte N (2004) Chem Rev 104:5931
Neuse EW (2005) J Inorg Organomet Polym Mater 15:3
Schatzschneider U, Metzler-Nolte N (2006) Angew Chem Int Ed 45:1504
Jaouen G, Top S, Vessières A, Leclercq G, McGlinchey MJ (2004) Curr Med Chem 11:2505
Vessières A, Top S, Beck W, Hillard E, Jaouen G (2006) Dalton Trans 529:19
Tabbi G, Cassino C, Cavigiolio G, Colangelo D, Ghiglia A, Viano I, Osella D (2002) J Med Chem 45:5786
Tamura H, Miwa M (1997) Chem Lett 26:1177
Simenel AA, Morozova EA, Snegur LV, Zykova SI, Kachala VV, Ostrovskaya LA, Bluchterova NV, Fomina MM (2009) Appl Organomet Chem 23:219
Dehaen W, Becher J (1993) Acta Chem Scand 47:244
Funicello M, Spagnolo P, Zanirato P (1993) Acta Chem Scand 47:231
Scriven EFV, Turnbull K (1988) Chem Rev 88:297 and references cited therein
Svenstrup N, Simonsen KB, Thorup N, Brodersen J, Dehaen W, Becher J (1999) J Org Chem 64:2814 and references cited therein
Meyer F (2006) Eur J Inorg Chem 3789
Burzlaff N, Hegelmann I, Weibert B (2001) J Organomet Chem 626:16
Joksovic MD, Markovic V, Juranic ZD, Stanojkovic T, Jovanovic LS, Damljanovic IS, Szécsényi KM, Todorovic N, Trifunovic S, Vukicevic RD (2009) J Organomet Chem 694:3935
Zhang Q, Song WLi, Showkot Hossain AM, Liu ZD, Hu GJ, Tian YP, Wu JY, Jin BK, Zhou HP, Yanga JX, Zhanga SY (2011) Dalton Trans 40:3510
Boev VI, Snegur LV, Babin VN, Nekrasov YuS (1997) Russ Chem 66:677
Sachivko AV, Tverdokhlebov VP, Tselinskii IV (1986) Russ J Org Chem 22:206
Sachivko AV, Tverdokhlebov VP, Tselinskii IV (1986) Russ J Org Chem 22:1112
Shirokobokov IYu, Sachivko AV, Tverdokhlebov VP, Ostrovskii VA, Tselinskii IV, Koldobskii GI (1986) Russ J Org Chem 22:1763
Basu PK, Lopez AGC, Font-Bardia M, Calvet T (2009) J Organomet Chem 694:3633
Togni A, Hayashi T (eds) (1995) Ferrocenes homogeneous catalysis organic synthesis materials science. VCH, Weinheim
Stepnicka P (ed) (2008) Ligands, materials and biomolecules. Ferrocene, Wiley
Arrayas RG, Adrio J, Carretero JC (2006) Angew Chem Int Ed 45:7674
Pou D, Platero-Prats AE, Perez S, Lopez C, Solans X, Font-Bardia M, van Leeuwen PWNM, van Strijdonck GPF, Freixa Z (2007) J Organomet Chem 692:5017
Perevalova EG, Reshetova MD, Grandberg KI (1983) Methods of organoelement chemistry. Ferrocene, Moscow, p 544
Rockett BW, Marr G (1972) J Organomet Chem 45:389
Rockett BW, Marr G (1991) J Organomet Chem 416:327
Cais M (1966) Organomet Chem Rev 1:435
Watts WE (1979) Organomet Chem Libr 7:399
Watts WE (1983) Ind J Phys 66B:1
Koridze AA (1986) Russ Chem 55:277
Rybinskaya MI (1992) Russ Chem Bull 1083
Osipova EYu, Simenel AA, Rodionov AN, Kachala VV (2012) Russ J Org Chem 48:1449
DeWald HA, Nordin IC, L’Italien YJ, Parcel RF (1973) J Med Chem 16:1346
Canonne P, Foscolos G, Harder R (1979) J Organomet Chem 178:331
Weliky N, Gould ES (1957) J Am Chem Soc 79:2742
Rodionov AN, Simenel AA, Korlyukov AA, Kachala VV, Peregudova SM, Zherebker KYa, Osipova EYu (2011) J Organomet Chem 696:2108
APEX II (2005) Bruker-AXS, 5465 East Cheryl Parkway Madison, Wisconsin 53711-5373, USA
Blessing RH (1995) Acta Cryst A51:33
Sheldrick GM (2015) Acta Cryst A71:3
Sheldrick GM (2015) Acta Cryst C71:3
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339
Moskalenko AI, Boeva AV, Boev VI (2011) Russ J Gen Chem 81:521
Burckhardt U, Baumann M, Trabesinger G, Gramlich V, Togni A (1997) Organometallics 16:5252
Chabert N, Jacquet L, Marzin C, Tarrago G (1995) New J Chem 19:443
Polyakov BV, Tverdokhlebov VP, Tselinskii IV, Bakstova NM, Frolova GM (1983) J Gen Chem 53:1847
Lachan M, Lapich V (1972) Croat Chem Acta 44:317
Kulikov VN, Nikitin OM, Borisov YuA, Makarov AS, Rodionov AN, Nikulin RS, Kovalenko LV, Belousov YuA (2014) Russ Chem Bull 63:2255
Acknowledgements
The reported study was funded by the Russian Foundation for Basic Research (RFBR), according to the research project No. 16-33-60163 (mol_a_dk).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodionov, A.N., Gerasimova, M.D., Osipova, E.Y. et al. Synthesis of bis-ferrocenylpyrazoles via ferrocenylalkylation reaction. Monatsh Chem 148, 925–932 (2017). https://doi.org/10.1007/s00706-016-1895-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1895-3