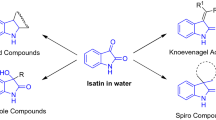
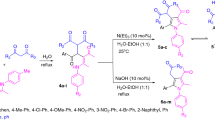
Abstract
Many aromatic compounds containing the isatin moiety have interesting applications in biology, industry, and drugs. Synthesis of different forms of these scaffolds, with a glance to green chemistry, has been performed in water as the reaction medium. This review describes the water-mediated preparation of various aromatic compounds representing isatin derivatives.
Graphical abstract


Similar content being viewed by others
References
Erdmann OL (1840) J Prakt Chem 19:321
Laurent A (1840) Ann Chim Phys 3:393
Guo Y, Chen F (1986) Zhongcaoyao 17:8. Chem Abstr 104:213068f
Yoshikawa M, Murakami T, Kishi A, Sakurama T, Matsuda H, Nomura M, Matsuda H, Kubo M (1998) Chem Pharm Bull 46:886
Bergman J, Lindstorm JO, Tilstam U (1985) Tetrahedron 41:2879
Wei L, Wang Q, Liu X (1982) Yaowu Fenxi Zazhi 2:288
D’ischi M, Palumbo A, Prota G (1988) Tetrahedron 44:6441
Palumbo A, D’ischi M, Misurace G, Prota G (1989) Biochim Biophys Acta 990:297
Halket JM, Watkins PJ, Przyborowska A, Goodwin BL, Clow A, Glower V, Sandler MJ (1991) Chromatogr 562:279
Pandeya SN, Smitha S, Jyoti M, Sridhar SK (2005) Acta Pharm 55:27
Pakravan P, Kashanian S, Khodaie MM, Harding FJ (2013) Pharmacol Rep 65:313
Gurkok G, Altanlar N, Suzen S (2009) Chemotherapy 55:15
Kang IJ, Wang LW, Hsu TA, Yueh A, Lee CC, Lee YC, Lee CY (2011) Bioorg Med Chem Lett 21:1948
Sridhar SK, Pandeya SN, Stables JP, Ramesh A (2002) Eur J Pharm Sci 16:129
Supriya M, Nilanjan P, Neeraj K (2010) Pharma Res 3:51
Panneerselvam P, Ravi Sankar R, Kumarasamy M, Ramesh Kumar N (2010) Pharma Chem 2:28
Saragapani M, Reddy V (1997) Indian J Pharm Sci 59:105
Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R (2010) Eur J Med Chem 45:1374
Nataraj KS, Venkateshwara Rao J, Jayaveera KN (2010) J Pharm Res 3:863
Pandeya S, Sriram D, Nath G, De Clercq E (1999) Eur J Pharm Sci 9:25
Pawar Y, Sonawane A, Nagle P, Mahulikar P, More D (2011) Int J Curr Pharm Res 3:47
Vine KL, Locke JM, Ranson M, Pyne SG, Bremner JB (2007) Bioorg Med Chem 15:931
Hall MD, Salam NK, Hellawell JL, Fales HM, Kensler CB, Ludwig JA, Szakács G (2009) J Med Chem 52:3191
Yadav M, Sharma U, Yadav PN (2013) Egypt J Pet 22:335
Chen G, Su HJ, Zhang M, Huo F, Zhang J, Hao XJ, Zhao JR (2012) Chem Cent J 6:90
Ramachandran S (2011) Int J Res Pharm Chem 1:289
Sharma S, Gangal S, Rauf A (2008) Rasayan J Chem 1:693
Grieco PA (1998) Organic synthesis in water. Blackie Academic & Professional, London
Li CJ (1993) Chem Rev 93:2023
Lindstrom UM (2002) Chem Rev 102:2751
Lindstrom UM (2007) Organic reactions in water: principles, strategies and applications. Blackwell Publishing, Oxford
Rideout DC, Breslow R (1980) J Am Chem Soc 102:7816
Chandrasekhar J, Shariffskul S, Jorgensen WL (2002) J Phys Chem B 106:8078
Pirrung MC (2006) Chem Eur J 12:1312
Otto S, Blokzijl W, Engberts JBFN (1994) J Org Chem 59:5372
Narayan S, Muldoon J, Finn MG, Fokin VV, Kolb HC, Sharpless KB (2005) Angew Chem Int Ed 44:3275
Riyaz Sd, Indrasena A, Naidu A, Dubey PK (2014) Indian J Chem Sec B 53:120
Naeimi H, Rashid Z, Zarnani AH, Ghahremanzadeh R (2014) New J Chem 38:348
Pore DM, Hegade PG, Mane MM, Patil JD (2013) RSC Adv 3:25723
Rai P, Srivastava M, Singh J, Singh J (2013) RSC Adv 3:18775
Karamthulla S, Pal S, Khan MN, Choudhury LH (2013) RSC Adv 3:15576
Ghahremanzadeh R, Rashid Z, Zarnani AH, Naeimi H (2013) Appl Catal A 467:270
Jalili Baleh L, Mohammadi N, Khoobi M, Mámani L, Foroumadi A, Shafiee A (2013) Helv Chim Acta 96:1601
Debnath K, Pathak S, Pramanik A (2013) Tetrahedron Lett 54:4110
Wang GD, Zhang XN, Zhang ZH (2013) J Heterocycl Chem 50:61
Alizadeh A, Mikaeili A, Firuzyar T (2012) Synthesis 44:1380
Sarrafi Y, Alimohammadi K, Sadatshahabi M, Norozipoor N (2012) Monatsh Chem 143:1519
Hosseini Sarvari M, Tavakolian M (2012) Appl Catal A 441–442:65
Rahmati A, Khalesi Z (2012) Tetrahedron 68:8472
Rineh A, Khalilzadeh MA, Hashemi MM, Rajabi M, Karimi F (2012) J Heterocycl Chem 49:789
Rahmati A, Vakili K (2012) HeIv Chim Acta 95:1126
Balamurugan K, Perumal S, Menendez JC (2011) Tetrahedron 67:3201
Dabiri M, Noroozi Tisseh Z, Nobahar M, Bazgir A (2011) Helv Chim Acta 94:824
Oskooie A, Heravi M, Karimi N, Hamidi H (2011) Synth Commun 41:3344
Chen H, Shi D (2010) J Comb Chem 12:571
Ghahremanzadeh R, Amanpour T, Sayyafi M, Bazgir A (2010) J Heterocycl Chem 47:421
Dabiri M, Bahramnejad M, Baghbanzadeh M (2009) Tetrahedron 65:9443
Jadidi Kh, Ghahremanzadeh R, Bazgir A (2009) J Comb Chem 11:341
Angelica G, Correa RJ, Garden SJ, Tomasini C (2009) Tetrahedron Lett 50:814
Alimohammadi K, Sarrafi Y, Tajbakhsh M (2008) Monatsh Chem 139:1037
Safaei HR, Shioukhi N, Shekouhy M (2013) Monatsh Chem 144:1855
Saluja P, Aggarwal K, Khurana JM (2013) Synth Commun 43:3239
Jin Ming Y, Lin W, Dong F, Jian C, Shunjun J (2013) Chin Sci Bull 58:2944
Mohammadi Ziarani G, Lashgari N, Badiei N, Shakiba M (2013) Chemija 24:142
Ramesh K, Murthy SN, Karnakar K, Negeswar YVD (2011) Tetrahedron Lett 52:4734
Entezari M, Hekmati M, Hekmat Sh, Ardestani Javadi E (2013) World Appl Sci J 21:1421
Paul S, Das AR (2013) Tetrahedron Lett 54:1149
Sridhar R, Srinivaas B, Madhar B, Reddy VP, Negeswar YVD, Rao KR (2009) Can J Chem 87:1704
Arya AK, Kumar M (2011) Green Chem 13:1332
Narasimhulu M, Lee YR (2011) Tetrahedron 67:9627
Wang LM, Jiao N, Qiu J, Yu JJ, Liu JQ, Guo FL, Liu Y (2010) Tetrahedron 66:339
Zhu SL, Ji SJ, Zhang Y (2007) Tetrahedron 63:9365
Quiroga J, Portillo S, Perez A, Galvez J, Abonia R, Insuasty B (2011) Tetrahedron Lett 52:2664
Rahmati A, Kenarkoohi T, Khavasi HR (2012) ACS Comb Sci 14:657
Mohammad Ziarani G, Badiei A, Mousavi S, Lashgari N, Shahbazi A (2012) Chin J Catal 33:1832
Baharfar R, Azimi R (2014) Synth Commun 44:89
Ghahramanzadeh R, Sayyafi M, Ahadi S, Bazgir A (2009) J Comb Chem 11:393
Kumar VP, Reddy VP, Sridhar R, Sriniras B, Narender M, Rao KR (2008) J Org Chem 73:1646
Hongyun G, Jinjin T (2011) Chinese J Org Chem 31:1752
Thakur BP, Sirisha K, Sarma AVS, Meshram HM (2014) Tetrahedron Lett 55:2459
Meshram HM, Rao NN, Kumar NS, Rao LC (2012) Pharma Chem 4:1355
Bazgir A, Ahadi S, Ghahremanzadeh R, Khavasi HR, Mirzaei P (2010) Ultrason Sonochem 17:447
Ahadi S, Ghahremanzadeh R, Mirzaei P, Bazgir A (2009) Tetrahedron 65:9316
Rajabi Khorrami A, Faraji F, Bazgir A (2010) Ultrason Sonochem 17:587
Baharfar R, Shariati N (2014) C R Chimie 17:413
Kumar A, Chimni SS (2013) Tetrahedron 69:5197
Rahmati A, Rezayan AH, Alizadeh M, Nikbakht A (2013) J Iran Chem Soc 10:521
Jing C, Shi T, Xing D, Guo X, Hu WH (2013) Green Chem 15:620
Shekouhy M (2012) Catal Sci Technol 2:1010
Karimi N, Oskooie H, Heravi MM, Saeedi M, Zakeri M, Tavakoli N (2011) Chin J Chem 29:321
Kumar A, Tripathi VD, Kumar P (2011) Green Chem 13:51
Paladhi S, Bhati M, Panda D, Dash J (2014) J Org Chem 79:1473
Thakur PB, Meshram HM (2014) RSC Adv 4:5343
Thakur PB, Meshram HM (2014) RSC Adv 4:6019
Jarrahpour AA, Khalili D (2006) Molecules 11:59
Lv Q, Fang L, Wang P, Lu C, Yan F (2013) Monatsh Chem 144:391
Ramireddy N, Zhao JCG (2014) Tetrahedron Lett 55:706
Gomes JC, Sirvent J, Moyano A, Rodrigues MT Jr, Coelha F (2013) Org Lett 15:5838
Shanthi G, Lakshmi NV, Perumal PT (2009) Arkivoc (x):121
Nasseri MA, Sadeghzadeh SM (2013) J Iran Chem Soc 10:1047
Raj M, Veerasamy N, Singh VK (2010) Tetrahedron Lett 51:2157
Yu C, Lyu H, Cai Y, Miao X, Miao Z (2013) RSC Adv 3:18857
Dandia A, Jain AK, Bhati DS (2011) Synth Commun 41:2905
Rajabi Khorrami A, Faraji F, Bazgir A (2011) Ultrason Sonochem 18:635
Kowsari E, Mallakmohammadi M (2011) Ultrason Sonochem 18:447
Dandia A, Singh R, Bhaskaran S, Samant SD (2011) Green Chem 13:1852
Dandia A, Singh R, Joshi J, Maheshwarib S, Soni P (2013) RSC Adv 3:18992
Arya K, Rawat DS, Sasai H (2012) Green Chem 14:1956
Wu Q, Feng H, Guo DD, Yi MS, Wang XH, Jiang B, Tu SJ (2013) J Heterocyl Chem 50:599
Meshram HM, Rao NN, Rao LC, Kumar NS (2012) Tetrahedron Lett 53:3963
Panda SS, Jain SC (2012) Monatsh Chem 143:1187
Bandyopadhyay D, Banik A, Bhatta S, Banik BK (2009) Heterocycl Commun 15:121
Flores M, Pena J, Garcia-Garcia P, Carrido NM, Diez D (2013) Curr Org Chem 17:1757
Deb ML, Bhuyan PJ (2009) Synth Commun 39:2240
Hasaninejad A, Shekouhy M, Mohammadizadeh MR, Zare A (2012) RSC Adv 2:6174
Rehn S, Bergman J, Stensland B (2004) Eur J Org Chem 2004:413
Chen G, Yong J, Gao S, He H, Li S, Di Y, Chang Y, Lu Y, Hao X (2012) Mol Divers 16:151
Nair V, Sheela KC, Rath NP, Eigendorf GK (2000) Tetrahedron Lett 41:6217
Ganguly AK, Seah N, Popov V, Wang CH, Kuang R, Saksena AK, Pramanik BN, Chan TM, Mcphil AT (2002) Tetrahedron Lett 43:8981
Kumar RS, Rajesh SM, Perumal S, Banerjee D, Yogeeswari P, Sriram D (2010) Eur J Med Chem 45:411
Hazra A, Paira P, Sahu KB, Naskar S, Saha P, Paira R, Mondal Sh, Maity A, Luger P, Weber M, Mondal NB, Banerjee S (2010) Tetrahedron Lett 51:1585
Subramaniyan G, Raghunathan R, Nethaji M (2002) Tetrahedron 58:9075
Babu ARS, Raghunathan R, Goyatri G, Sastry GN (2006) J Heterocycl Chem 43:1467
Shintani R, Inoue M, Hayashi T (2006) Angew Chem Int Ed 45:3353
Kumar MA, Krishna AB, Babu BH, Reddy CB, Reddy CS (2008) Synth Commun 38:3456
Ivachtchenko AV, Kobak VV, Ilyn AP, Khvat AV, Kysil VM, Williams CT, Kuzovkova JA, Kravchenko DV (2005) J Comb Chem 7:227
Chen T, Xu XP, Ji SJ (2010) J Comb Chem 12:659
Elinson MN, Ilovaisky AI, Merkulova VM, Zaimovskaya TA, Nikishin GI (2012) Mendeleev Commun 22:143
Rad Moghadam K, Youseftabar Miri L (2010) Bhuyan 13:1969
Guo RY, An ZM, Mo LP, Yang ST, Liu HX, Wang SX, Zhang ZH (2013) Tetrahedron 69:9931
Fadda AA, El-Mekabaty A, Elattar KhM (2013) Synth Commun 43:2685
Wu C, Shen R, Chen J, Hu C (2013) Bull Korean Chem Soc 34:2431
Kumar A, Chimni SS (2013) Eur J Org Chem 4780
Alcaide B, Almendros P, Aragoncillo C (2010) Eur J Org Chem 2845
Dandia A, Jain AK, Laxkar AK, Bhati DS (2013) Tetrahedron Lett 54:3180
Guo RY, An ZM, Mo LP, Wang RZ, Liu HX, Wang SX, Zhang ZH (2013) ACS Comb Sci 15:557
Hara N, Nakamura S, Shibata N, Toru T (2009) Chem Eur J 15:6790
Liu Y, Gao P, Wang J, Sun Q, Ge Z, Li R (2012) Synlett 23:1031
Khorshidi A, Tabatabaeian Kh (2011) J Serb Chem Soc 76:1347
Xue F, Zhang S, Liu L, Duan W, Wang W (2009) Chem Asia J 4:1664
Nakamura S, Hara N, Nakashima H, Kubo K, Shibata N, Toru T (2008) Chem Eur J 14:8079
Acknowledgments
The authors express their gratitude for financial support from Alzahra University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikoofar, K., Dizgarani, S.M. A concise synthesis of isatin-based aromatic compounds in water as the reaction medium resulting in an approach to green chemistry. Monatsh Chem 146, 1161–1204 (2015). https://doi.org/10.1007/s00706-015-1439-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1439-2