Abstract
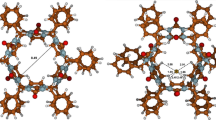
By using quantum mechanical DFT calculations, the most probable structures of the bambus[6]uril—CH3SO −3 and bambus[6]uril—CF3SO −3 anionic complex species were derived. In these two complexes having C 3 symmetry, each of the considered anions, included in the macrocyclic cavity, is bound by 12 weak hydrogen bonds between methine hydrogen atoms on the convex face of glycoluril units and the respective anion.
Graphical abstract




Similar content being viewed by others
References
Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L (2005) Angew Chem Int Ed 44:4844
Lee JW, Samal S, Selvapalam N, Kim HJ, Kim K (2003) Acc Chem Res 36:621
Liu S, Ruspic C, Mukhopadhyay P, Chakrabarti S, Zavalij PY, Isaacs L (2005) J Am Chem Soc 127:15959
Freeman WA, Mock WL, Shih NY (1981) J Am Chem Soc 103:7367
Mock WL, Shih NY (1983) J Org Chem 48:3618
Mock WL, Shih NY (1986) J Org Chem 51:4440
Mock WL, Shih NY (1988) J Am Chem Soc 110:4706
Mock WL, Shih NY (1989) J Am Chem Soc 111:2697
Isobe H, Tomita N, Lee JW, Kim HJ, Kim K, Nakamura E (2000) Angew Chem Int Ed 39:4257
Isobe H, Sota S, Lee JW, Kim HJ, Kim K, Nakamura E (2005) Chem Commun 1549–1551
Tan Y, Choi S, Lee JW, Ko YH, Kim K (2002) Macromolecules 35:7161
Márquez C, Hudgins RR, Nau WM (2004) J Am Chem Soc 126:5806
Buschmann HJ, Mutihac L, Mutihac RC, Schollmeyer E (2005) Thermochim Acta 430:79
Buschmann HJ, Schollmeyer E, Mutihac L (2003) Thermochim Acta 399:203
Miyahara Y, Goto K, Oka M, Inazu T (2004) Angew Chem Int Ed 43:5019
Li Y, Li L, Zhu Y, Meng X, Wu A (2009) Cryst Growth Des 9:4255
Buschmann HJ, Zielesny A, Schollmeyer E (2006) J Incl Phenom Macrocycl Chem 54:181
Buschmann HJ, Cleve E, Schollmeyer E (2005) Inorg Chem Commun 8:125
Svec J, Necas M, Sindelar V (2010) Angew Chem Int Ed 49:2378
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Becke AD (1993) J Chem Phys 98:5648
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision C.01. Gaussian Inc, Wallingford
Kříž J, Dybal J, Makrlík E, Budka J (2008) J Phys Chem A 112:10236
Kříž J, Dybal J, Makrlík E, Budka J, Vaňura P (2009) J Phys Chem A 113:5896
Kříž J, Toman P, Makrlík E, Budka J, Shukla R, Rathore R (2010) J Phys Chem A 114:5327
Makrlík E, Toman P, Vaňura P (2012) Monatsh Chem 143:199
Makrlík E, Toman P, Vaňura P (2013) Monatsh Chem 144:919
Boys SF, Bernardi F (1970) Mol Phys 19:553
van Duijneveldt FB, van Duijneveldt-van de Rijdt JGCM, van Lenthe JH (1994) Chem Rev 94:1873
Acknowledgments
This work was supported by the Grant Agency of Faculty of Environmental Sciences, Czech University of Life Sciences, Prague, Project No.: 42900/1312/3114 “Environmental Aspects of Sustainable Development of Society”, and by the Czech Ministry of Education, Youth, and Sports (Project MSM 6046137307).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makrlík, E., Toman, P. & Vaňura, P. Theoretical study on the complexation of bambus[6]uril with the methanesulfonate and trifluoromethanesulfonate anions. Monatsh Chem 146, 1609–1612 (2015). https://doi.org/10.1007/s00706-015-1417-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1417-8