Abstract
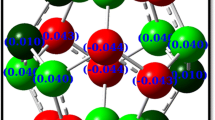
We are focusing our calculations on the structural stabilities and electronic properties of six novel C20−n Ge n heterofullerenes, with n = 5–10, at B3LYP/6-311++G** and B3LYP/AUG-cc-pVTZ levels. Vibrational frequency calculations on C20 and its six C20−n Ge n analogs show them as true minima. In contrast to identical bonds in the former, contractions of C=C double bonds are encountered at the expense of longer C–Ge bonds in heterofullerenes. Band gaps (ΔE HOMO–LUMO) of heterofullerenes become narrower as n increases. As to band gaps, C14Ge6 immerges with the highest ΔE HOMO–LUMO = 1.81 eV. Hence, it is predicted to be most stable against electronic excitation. It has C i symmetry and contains four germanium atoms in equatorial and two at the cap positions. On the other hand, C15Ge5 appears with the lowest ΔE HOMO–LUMO = 1.25 eV. It has C 5v symmetry and contains five alternating germanium atoms in equatorial position. So, C15Ge5 is predicted to orchestrate a higher conductivity and charge transfer, making it a possible candidate for hydrogen storage. Assuming the binding energy (E b) as a criterion of stability, the more stable species turn out to be both C20 parent fullerene, and C15Ge5 heterofullerene with E b = 8.0, and 7.0 eV/atom, respectively.
Graphical Abstract






Similar content being viewed by others
References
Lu X, Chen Z (2005) Chem Rev 105:3643
Alasak T, Nagase S (2001) Endofullerenes: a new family of carbon clusters. Kluwer Academic, Dordrecht
Luo J, Peng LM, Xue ZQ, Wu JL (2004) J Chem Phys 120:7998
Chen Z, Heine T, Jiao H, Hirsch A, Thiel W, Schleyer PvR (2004) Chem Eur J 10:963
Slanina Z, Adamowicz L (1993) Fullerene Sci Technol 1:1
Slanina Z, Adamowicz L (1992) Thermochim Acta 205:299
Slanina Z, Adamowicz L (1993) J Mol Struct (THEOCHEM) 281:33
Handschuh H, Gantefor G, Kessler B, Bechthold PS, Eberhardt W (1995) Phys Rev Lett 74:1095
Ott AK, Rechtsteiner GA, Felix C, Hampe O, Jarrold MF, Duyne RPV, Raghavachari K (1998) J Chem Phys 109:9652
Domene MC, Fowler PW, Mitchell D, Seifert G, Zerbetto F (1997) J Phys Chem A 101:8339
Hirsch A, Chen Z, Jiao H (2000) Angew Chem Int Ed 39:3915
Galli G, Gygi F, Golaz JC (1998) Phys Rev B 57:1860
Bertau M, Wahl F, Weiler A, Scheumann K, Worth J, Keller M, Prinzbach H (1997) Tetrahedron 53:10029
Prinzbach H, Weller A, Landenberger P, Wahl F, Worth J, Scott LT, Gelmont M, Olevano D, Issendorff B (2000) Nature 407:60
Buhl M, Hirsch A (2006) Chem Rev 106:5191
Prinzbach H, Weller A, Landenberger P, Wahl F, Worth J, Scott LT, Gelmont M, Olevano D, Issendorff BV (2000) Nature 407:60
Slanina Z, Zhao X, Chiang LY, Osawa E (1999) Int J Quantum Chem 74:343
Alder RW, Blake M, Oliva JM (1999) J Phys Chem A 103:11200
Huda MN, Ray AK (2008) Chem Phys Lett 457:124
Tang C, Zhu W, Deng K (2009) J Mol Struct 909:43
Froudakis GE (2001) Nano Lett 1:531
Menon M, Richter E, Mavrandonakis A, Froudakis GE, Andriotis AN (2004) Phys Rev B 69:115322
Mavrandonakis A, Froudakis GE, Schnell M, Muhlhauser M (2003) Nano Lett 3:1481
Mpourmpakis G, Froudakis GE, Lithoxoos GP, Samios J (2006) Nano Lett 6:1581
Becke AD (1988) Phys Rev A 38:3098
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Znkrzewski VG, Montgomery GA, Startmann RE Jr, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pamelli C, Adamo G, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokoma D, Malick K, Rubuck AD, Raghavachari K, Foresman JB, Cioslawski J, Oritz JV, Stlefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Comperts R, Martin RL, Fox PJ, Keith T, Al-laham MA, Peng CY, Akkara AN, Gonzales CG, Combe MC, Gill PMW, Johnson B, Chem W, Wong MW, Andres JL, Gonzales C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian, vol 98. Gaussian Inc, Pittsburgh
Hariharan PC, Pople JA (1974) Mol Phys 27:209
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) J Chem Phys 77:3654
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PvR (1983) J Comput Chem 4:294
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265
Krishna R, Frisch MJ, Pople JA (1980) J Chem Phys 72:4244
Hehre WJ, Radom L, PvR Schleyer, Pople JA (1986) Ab Initio molecular orbital theory. Wiley, New York
Glendening ED, Reed AE, Carpenter JE, Weinhold F. NBO Version 3.1
Schleyer PvR, Maerker C, Dransfeld A, Jiao H, Hommes NJRvE (1996) J Am Chem Soc 118:6317
Chen Z, Ma K, Zhao H, Pan Y, Zhao X, Tang A, Feng J (1999) J Mol Struct (THEOCHEM) 466:127
Mizorogi N, Aihara J-I (2003) Phys Chem Chem Phys 5:3368
Watanabe M, Ishimaru D, Mizorogi N, Kiuchi M, Aihara J-I (2005) J Mol Struct (THEOCHEM) 726:11
Yang Z, Xu X, Wang G, Shang Z, Cai Z, Pan Y, Zhao X (2002) J Mol Struct (THEOCHEM) 618:191
Acknowledgments
The authors wish to gratefully thank Dr. M. Ghambarian, and H. Zandi for many useful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
706_2014_1388_MOESM1_ESM.doc
Supporting Information available: Full references for Gaussian 98, xyz coordinates, and all energies given as kcal/mol are expressed in the SI unit kJ/mol for optimized structures studied in this work (DOC 65 kb)
Rights and permissions
About this article
Cite this article
Koohi, M., Kassaee, M.Z., Ghavami, M. et al. C20−n Ge n heterofullerenes (n = 5–10) on focus: a density functional perspective. Monatsh Chem 146, 1409–1417 (2015). https://doi.org/10.1007/s00706-014-1388-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1388-1