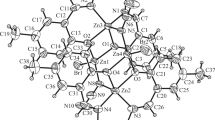
Abstract
Two pentadentate carbohydrazone ligands, H2L1 and H2L2, were prepared by the reaction of the bifunctional compound carbohydrazide with 2-hydroxy-5-nitrobenzaldehyde and 2-hydroxy-1-naphthaldehyde, respectively. Reactions of the ligands with oxovanadium(IV), cerium(III), thorium(IV), and dioxouranium(VI) ions yielded binary complexes. Reactions of the ligands with the dioxouranium(VI) ion in the presence of secondary ligands (8-hydroxyquinoline, glycine, salicylaldehyde, or benzoylacetone) yielded ternary complexes. The ligands and metal complexes were characterized by different techniques such as elemental and thermal analyses, IR, 1H and 13C NMR, electronic, ESR, mass spectra, and powder XRD as well as magnetic susceptibility and conductivity measurements. The coordinating sites are phenolic oxygen, azomethine nitrogen, and carbonyl oxygen. In complexes, the ligands act as dibasic pentadentate except ternary dioxouranium(VI) complexes, obtained using glycine or benzoylacetone, in which the ligands act as monobasic pentadentate. The XRD patterns for the H2L1 ligand, its binary dioxouranium(VI) complex, and its 8-hydroxyquinoline ternary complex indicate crystalline nature and the grain size was estimated. The H2L1 ligand and its binary complex have triclinic systems while the ternary complex has a monoclinic system with different unit-cell parameters. The ligands and some of their metal complexes showed antimicrobial activity toward some Gram-positive and Gram-negative bacteria, yeast (Candida albicans), and fungus (Aspergillus fumigatus), and MIC values were determined. The DNA binding properties of the oxovanadium(IV) complexes of H2L1 and H2L2 ligands were investigated by electronic absorption spectroscopy and viscosity measurements. The results indicated that these complexes bind to DNA via an intercalation binding mode with an intrinsic binding constant K b of 2.55 × 104 and 3 × 104 M−1, respectively.
Graphical Abstract









Similar content being viewed by others
References
Harinath Y, Reddy DHK, Kumar BN, Apparao CH, Seshaiah K (2013) Spectrochim Acta A 101:264
Sedaghat T, Tahmasbi L, Motamedi H, Reyes-Martinez R, Morales-Morales D (2013) J Coord Chem 66:712
Abu El-Reash GM, El-Gammal OA, Radwan AH (2014) Spectrochim Acta A 121:259
Abu El-Reash GM, El-Gammal OA, Ghazy SE, Radwan AH (2013) Spectrochim Acta A 104:26
El-Gammal OA, Abu El-Reash GM, Ghazy SE, Radwan AH (2012) J Mol Struct 1020:6
Eswaran S, Adhikari AV, Pal NK, Chowdhury IH (2010) Bioorg Med Chem Lett 20:1040
Reddy KH, Reddy PS, Babu PR (2000) Trans Met Chem 25:505
Joshi JD, Sharma S, Patel G, Vora JJ (2002) Synth React Inorg Met Org Chem 32:1729
Subbaraj P, Ramu A, Raman N, Dharmaraja J (2014) Spectrochim Acta A 117:65
Dharmaraja J, Esakkidurai T, Subbaraj P, Shobana S (2013) Spectrochim Acta A 114:607
Aljahdali M, El-Sherif AA (2013) Inorg Chim Acta 407:58
Abu Ali H, Darawsheh MD, Rappocciolo E (2013) Polyhedron 61:235
Shobana S, Dharmaraja J, Selvaraj S (2013) Spectrochim Acta A 107:117
Sampath K, Sathiyaraj S, Raja G, Jayabalakrishnan C (2013) J Mol Struct 1046:82
Mashaly MM, El-Shafiy HF, El-Maraghy SB, Habib HA (2005) Spectrochim Acta A 61:1869
Mishra AP, Soni M (2008) Met Based Drugs 10:1
Dong Y, Narla RK, Sudbeck E, Uckun FM (2000) J Inorg Biochem 78:321
Noblia P, Vieites M, Parajon-Costa BS, Baran EJ, Cerecetto H, Draper P, Gonzalez M, Piro OE, Castellano EE, Azqueta A, Cerain AL, Monge-Vega A, Gambino D (2005) J Inorg Biochem 99:443
Szacilowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M, Stochel G (2005) Chem Rev 105:2647
Sakurai H, Kojitane Y, Yoshikawa Y, Kawabe K, Yasui H (2002) Coord Chem Rev 226:187
Cotton SA (1999) Polyhedron 18:1691
Meehan PR, Aris DR, Willey GR (1999) Coord Chem Rev 181:121
Drozdzynski J (2005) Coord Chem Rev 249:2351
Sessler JL, Melfi PJ, Pantos GD (2006) Coord Chem Rev 250:816
Bunzli J-CG, Piquet C (2002) Chem Rev 102:1897
Bunzli J-CG (2006) Acc Chem Res 39:53
Zhao P, Xu LC, Huang JW, Zheng KC, Liu J, Yu HC, Ji LN (2008) Biophys Chem 134:72
Dhar S, Nethaji M, Charkravarty AR (2005) Inorg Chem 44:8876
Kumar RS, Arunachalam S (2008) Biophys Chem 136:136
Alaghaz AMA, El-Sayed BA, El-Henawy AA, Ammar RAA (2013) J Mol Struct 1035:83
Karastogianni S, Dendrinou-Samara C, Ioannou E, Raptopoulou CP, Hadjipavlou-Litina D, Girousi S (2013) J Inorg Biochem 118:48
Pathan AH, Bakale RP, Naik GN, Frampton CS, Gudasi KB (2012) Polyhedron 34:149
Kulkarni NV, Kamath A, Budagumpi S, Revankar VK (2011) J Mol Struct 1006:580
Benítez J, de Queiroz AC, Correia I, Alves MA, Alexandre-Moreira MS, Barreiro EJ, Lima LM, Varela J, González M, Cerecetto H, Moreno V, Pessoa JC, Gambino D (2013) Eur J Med Chem 62:20
Li L, Guo Z, Zhang Q, Xu T, Wang D (2010) Inorg Chem Commun 13:1166
Banik B, Somyajit K, Koley D, Nagaraju G, Chakravarty AR (2012) Inorg Chim Acta 393:284
Prasad P, Sasmal PK, Khan I, Kondaiah P, Chakravarty AR (2011) Inorg Chim Acta 372:79
Abu-Hussen AAA, Emara AAA (2004) J Coord Chem 57:973
Lever ABP (1984) Inorganic electronic spectroscopy, 2nd edn. Elsevier, Amsterdam
Shrivastav A, Singh NK, Tripathi P, George T, Dimmock JR, Sharma RK (2006) Biochimie 88:1209
Shebl M (2008) Spectrochim Acta A 70:850
Shebl M, Ibrahim MA, Khalil SME, Stefan SL, Habib H (2013) Spectrochim Acta A 115:399
Shebl M (2009) J Coord Chem 62:3217
Seleem HS, El-Shetary BA, Khalil SME, Mostafa M, Shebl M (2005) J Coord Chem 58:479
Shebl M, Khalil SME, Taha A, Mahdi MAN (2013) Spectrochim Acta A 113:356
Shebl M, Khalil SME, Taha A, Mahdi MAN (2012) J Am Sci 8:183
Soliman AA, Mohamed GG (2004) Thermochim Acta 421:151
Chandra S, Pundir M (2007) Spectrochim Acta A 68:833
Saif M, Mashaly MM, Eid MF, Fouad R (2012) Spectrochim Acta A 92:347
Shebl M, Khalil SME, Ahmed SA, Medien HAA (2010) J Mol Struct 980:39
Khalil SME, Shebl M, Al-Gohani FS (2010) Acta Chim Slov 57:716
Shebl M (2009) Spectrochim Acta A 73:313
Khalil SME, Seleem HS, El-Shetary BA, Shebl M (2002) J Coord Chem 55:883
Shebl M, Khalil SME, Al-Gohani FS (2010) J Mol Struct 980:78
McGlynn SP, Smith JK, Neely WC (1961) J Chem Phys 35:105
Jones LH (1958) Spectrochim Acta A 10:395
Selbin LHH, McGlynn SP (1963) J Inorg Nucl Chem 25:1359
El-Asmy AA, El-Gammal OA, Radwan HA, Ghazy SE (2010) Spectrochim Acta A 77:297
Shebl M (2014) Spectrochim Acta A 117:127
Geary WJ (1971) Coord Chem Rev 7:81
El-Metwally NM, El-Shazly RM, Gabr IM, El-Asmy AA (2005) Spectrochim Acta A 61:1113
Khalil SME, El-Shafiy HFO (2000) Synth React Inorg Met Org Chem 30:1817
Sarrano CJ, Bonadies JA (1986) J Am Chem Soc 108:4088
Ooi S, Nishizawa M, Matasuto K, Kuroya H, Saito K (1979) Bull Chem Soc Jpn 52:452
Shebl M, Seleem HS, El-Shetary BA (2010) Spectrochim Acta A 75:428
Abd El-Wahab ZH, Mashaly MM, Salman AA, El-Shetary BA, Faheim AA (2004) Spectrochim Acta A 60:2861
Tarafder MTH, Ali AM, Wong YW, Wong SH, Crouse KA (2001) Synth React Inorg Met Org Chem 31:115
Ferenc W, Dziewulska AW (2001) J Serb Chem Soc 66:543
Zheng YQ, Zhou LX, Lin JL, Wei DY (2002) Z Naturforsch 57b:1244
Khalil SME (2003) J Coord Chem 56:1013
Mashaly MM, Abd El-Wahab ZH, Faheim AA (2004) J Chin Chem Soc 51:1
Sun W, Yuan G, Liu J, Ma L, Liu C (2013) Spectrochim Acta A 106:275
Patel DA, Patel AA, Patel HS (2013) Arab J Chem. doi:10.1016/j.arabjc.2013.07.056
Zhang-Lin Y, Forissier M, Vedrine JC, Volta JC (1994) J Catal 145:267
Bencini A, Gattechi D (1990) EPR of exchange coupled systems. Springer, Berlin
Abou-Hussen AA, El-Metwally NM, Saad EM, El-Asmy AA (2005) J Coord Chem 58:1735
Yen TF (1969) Electron spin resonance of metal complexes, 1st edn. Plenum Press, New York
Mangalam NA, Kurup MRP (2009) Spectrochim Acta A 71:2040
Raman N, Kulandaisamy A, Jeyasubramanian K (2002) Synth React Inorg Met Org Chem 32:1583
Shebl M, Khalil SME, Taha A, Mahdi MAN (2012) J Mol Struct 1027:140
Seleem HS, El-Shetary BA, Shebl M (2007) Heteroatom Chem 18:100
Seleem HS, Emara AA, Shebl M (2005) J Coord Chem 58:1003
Quan CX, Bin LH, Bang GG (2005) Mater Chem Phys 91:317
Shirley R (2000) CRYSFIRE system for automatic powder indexing: user’s manual. The Lattice Press, Guildford
Thimmaiah KN, Lloyd WD, Chandrappa GT (1985) Inorg Chim Acta 106:81
Chakrabarti P (1993) J Mol Biol 234:463
Khan TA, Naseem S, Khan SN, Khan AU, Shakir M (2009) Spectrochim Acta A 73:622
Anbu S, Kandaswamy M, Suthakaran P, Murugan V, Varghese B (2009) J Inorg Biochem 103:401
Chosh K, Kumar P, Tyagi N, Singh UP, Coel N, Chakraborty A, Roy P, Baratto MC (2011) Polyhedron 30:2667
Wang XL, Chao H, Hong XL, Liu YJ, Ji LN (2005) Trans Met Chem 30:305
Rompel A, Fischer H, Meiwes D, Karentzopoulos KB, Diillinger R, Tuczek F, Witzel H, Krebs B (1999) J Biol Inorg Chem 4:56
Gao F, Chao H, Zhou F, Yuan Y-X, Peng B, Ji LN (2006) J Inorg Biochem 100:1487
Barton JK, Danishefsky AT, Goldberg JM (1984) J Am Chem Soc 106:2172
Uma V, Kanthimathi M, Weyhermuller T, Nair BU (2005) J Inorg Biochem 99:2299
Anbu S, Kandaswamy M (2011) Polyhedron 30:123
Mudasir, Yoshioka N, Inoue H (1999) J Inorg Biochem 77:239
Shivakumar L, Shivaprasad K, Revanasiddappa HD (2012) Spectrochim Acta A 97:659
Lu J, Guo H, Zeng X, Zhang Y, Zhao P, Jiang J, Zang L (2012) J Inorg Biochem 112:39
Leelavathy L, Anbu S, Kandaswamy M, Karthikeyan N, Mohan N (2009) Polyhedron 28:903
Lu JZ, Du YF, Guo HW (2011) J Coord Chem 64:1229
McCrate A, Carlone M, Nielsen M, Swavey S (2010) Inorg Chem Commun 13:537
Chaires JB, Dattagupta N, Crothers DM (1982) Biochemistry 21:3933
Satyanarayana S, Dabrowiak JC, Chaires JB (1992) Biochemistry 31:9319
Jin L, Yang P (1997) J Inorg Biochem 68:79
Satyanarayana S, Dabrowiak JC, Chaires JB (1993) Biochemistry 32:2573
Mabbs FE, Machin DI (1973) Magnetism and transition metal complexes. Chapman and Hall, London
Bauer AW, Kirby WWM, Sherris JC, Turck M (1966) Am J Clin Pathol 45:493
Rahman AU, Choudhary MI, Thomsen WJ (2001) Bioassay techniques for drug development. Harwood Academic Publishers, The Netherlands
Andrews JM (2001) J Antimicrob Chemother 48:5
Marmur V (1961) J Mol Biol 3:208
Reichmann ME, Rice SA, Thomas CA, Doty PJ (1954) J Am Chem Soc 76:3047
Cohen G, Eisenberg H (1969) Biopolymers 8:45
Barton JK, Goldberg JM, Kumar CV, Turro NJ (1986) J Am Chem Soc 108:2081
Acknowledgments
The Authors are grateful to Prof. Dr. M.M. Mashaly and Dr. M. Saif, providing facilities to carry out DNA studies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shebl, M., Khalil, S.M.E. Synthesis, spectral, X-ray diffraction, antimicrobial studies, and DNA binding properties of binary and ternary complexes of pentadentate N2O3 carbohydrazone ligands. Monatsh Chem 146, 15–33 (2015). https://doi.org/10.1007/s00706-014-1302-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1302-x