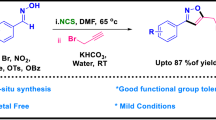
Abstract
The carbon-heteroatom bond formation is an important research field. Transition-metal-free synthesis of medicinally important heterocycles avoids products of transition metal contamination, and thus it is an environmentally friendly and cost-saving process. A transition-metal-free domino C-S/C-N formation for the synthesis of imidazobenzothiazines from 2-mercaptobenzimidazoles and 2-halobenzyl bromides is developed. The desired products were obtained in good to excellent yields. The mechanism of domino nucleophilic substitution (SN2) and nucleophilic aromatic substitution (SNAr) is proposed.
Graphical abstract

Similar content being viewed by others
References
Corma A, Leyva-Pérez A, Sabater MJ (2011) Chem Rev 111:1657
Noël T, Buchwald SL (2011) Chem Soc Rev 40:5010
Monnier F, Taillefer M (2008) Angew Chem Int Ed 47:3096
Beller M, Breindl C, Riermeier TH, Tillack A (2001) J Org Chem 66:1403
Prüger B, Hofmeister GE, Jacobsen CB, Alberg DG, Nielsen M, Jørgensen KA (2010) Eur J Chem 16:3783
Bonet A, Pubill-Ulldemolins C, Bo C, Gulyás H, Fernández E (2011) Angew Chem Int Ed 50:7296
Chen B, Gao T, Zhao M, Meng X, Li C (2011) Synlett 1281
Choi YL, Lim HS, Lim HJ, Heo J-N (2012) Org Lett 14:5102
Giernoth R, Bankmann D (2008) Eur J Org Chem 2881
Guo F, Wang L, Wang P, Yu J, Han J (2010) Asian J Org Chem 1:218
Li H, Wang L, Zhang Y, Wang J (2012) Angew Chem Int Ed 51:2943
Li Y, Studer A (2012) Angew Chem Int Ed 51:8221
Pirali T, Zhang F, Miller AH, Head JL, McAusland D, Greaney MF (2012) Angew Chem Int Ed 51:1006
Zhao J, Zhao Y, Fu H (2011) Angew Chem Int Ed 50:3769
Thomé I, Besson C, Kleine T, Bolm C (2013) Angew Chem Int Ed 52:7509
Lazer ES, Matteo MR, Possanza GJ (1987) J Med Chem 30:726
Abdel-Aziz HA, Gamal-Eldeen AM, Hamdy NA, Fakhr IMI (2009) Arch Pharm Chem Life Sci 342:230
Hosamani KM, Shingalapur RV (2011) Arch Pharm Chem Life Sci 11:311
Carcanague D, Shue Y-K, Wuonola MA, Uria-Nickelsen M, Joubran C, Abedi JK, Jones J, Kühler TC (2002) J Med Chem 45:4300
Sivakumar PM, Babu SKG, Doble M (2008) Chem Biol Drug Des 71:447
Vasan M, Neres J, Williams J, Wilson DJ, Teitelbaum AM, Remmel RP, Aldrich CC (2010) Chem Med Chem 5:2079
Setyan A, Sauvain J-J, Guillemin M, Riediker M, Demirdjian B, Rossi MJ (2010) Chem Phys Chem 11:3823
Shukla JS, Singh HH, Parmar SS (1969) J Prakt Chem 311:187
Clément M-J, Krishnan R, Elisabeth A, Flavio T, Curmi PA, Dulal P (2008) Biochemistry 47:13016
Bauer J, Kinast S, Burger-Kentischer A, Finkelmeier D, Kleymann G, Rayyan WA, Schröppel K, Singh A, Jung G, Wiesmüller K-H, Rupp S, Eickhoff H (2011) J Med Chem 54:6993
Narendar N, Velmathi S (2010) Tetrahedron Lett 50:5159
Deng H, Li Z, Ke F, Zhou X (2012) Chem Eur J 18:4840
Peter M, Knudsen LB, Wiberg FC, Carrr RD (1998) J Med Chem 41:5150
Okamoto O, Kobayashi K, Kawamoto H, Ito S, Satoh A, Kato T, Yamamoto I, Mizutani S, Hashimoto M, Shimizu A, Sakoh H, Nagatomi Y, Iwasawa Y, Takahashi H, Ishii Y, Ozaki S, Ohta H (2008) Bioorg Med Chem Lett 18:3278
Ma D, Yang J (2001) J Am Chem Soc 123:9706
Patru Samide A, Bibicu I (2008) Surf Interface Anal 40:944
Negm NA, Ghuiba FM, Mahmoud SA, Tawfik SM (2011) Eng Life Sci 11:496
Aljourani J, Raeissi K, Golozar MA (2009) Corros Sci 51:1836
Wang R, Qian W, Bao W (2012) Tetrahedron Lett 53:442
Wang Z, Yu B, Cui Y, Sun X, Bao W (2011) Chin J Chem 29:2769
Wang Z, Yu B, Zhang X, Sun X, Bao W (2011) Chin J Chem 29:2775
Patel BK (2010) J Comb Chem 12:754
Bao W, Liu Y, Lv X, Qian W (2008) Org Lett 10:3899
Caille S, Bercot EA, Cui S, Faul MM (2008) J Org Chem 73:2003
Huang A, Qiao Z, Zhang X, Yu W, Zheng Q, Ma Y, Ma C (2012) Tetrahedron 68:906
Qiu J-W, Zhang X-G, Tang R-Y, Zhong P, Li J-H (2009) Adv Synth Catal 351:2319
Ding Q, He X, Wu J (2009) J Comb Chem 11:587
Nicolaou KC, Edmonds DJ, Bulger PG (2006) Angew Chem Int Ed 45:7134
Dai C, Sun X, Tu X, Wu L, Zhan D, Zeng Q (2012) Chem Commun 48:5367
Sun X, Tu X, Dai C, Zhang X, Zhang B, Zeng Q (2012) J Org Chem 77:4454
Dong J, Wang Y, Xiang Q, Lv X, Weng W, Zeng Q (2013) Adv Synth Catal 355:692
Lv X, Xiang Q, Zeng Q (2014) Org Prep Proced Int 46:164
Zhan D, Li T, Wei H, Weng W, Ghandi K, Zeng Q (2013) RSC Adv 3:9325
Qiu D, Wei H, Zhou L, Zeng Q (2014) Appl Organomet Chem 28:109
Wei H, Li T, Zhou Y, Zhou L, Zeng Q (2013) Synthesis 45:3349
Bahrami K, Khodaei MM, Sheikh Arabi M (2010) J Org Chem 75:6208
Acknowledgments
We thank the National Natural Science Foundation of China (No. 21372034) and the cultivating program for excellent innovation team of Chengdu University of Technology.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tu, X., Zhou, L., Li, Z. et al. Transition-metal-free synthesis of imidazobenzothiazines via domino C-S/C-N bond formation. Monatsh Chem 145, 1925–1931 (2014). https://doi.org/10.1007/s00706-014-1264-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1264-z