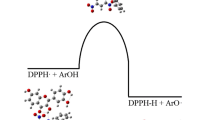
Abstract
Density functional theory was employed to investigate the molecular properties of two flavonoids, bractein and cernuoside, that serve as antioxidants. The B3LYP/6-311G** protocol was used for all computations. Investigations were performed in the gas phase and in two solvents with different polarity (water and benzene); the present work was devoted mainly to the determination of the O–H bond dissociation enthalpies and the ionization potentials of the examined compounds, since these quantities represent the most important parameters on which biological activity can be rationalized. The rotational energy of pyrogallol and catechol moieties together with highest occupied molecular orbital–lowest unoccupied molecular orbital and dipole moment analysis are reported for the two flavonoids. The present analysis also includes the spin density distribution for the radicals formed after H atom removal on each OH site of both flavonoids. The theoretical bond dissociation enthalpy values for these systems follow the same trend in gas and solvent phases. On the basis of computed bond dissociation enthalpy and ionization potential values, the most reactive system that is able to transfer an H-atom and electron transfer mechanism is found to be bractein followed by cernuoside. All these results suggest bractein to be a potential antioxidant similar to quercetin.
Graphical Abstract

.








Similar content being viewed by others
References
Robbins RJ (2003) J Agr Food Chem 51:2866
Macheix JJ, Fleuriet A, Billot J (1990) Fruit phenolics. CRC, Boca Raton
Havsteen B (1983) Biochem Pharmacol 23:1141
Cody V, Middleton EJR, Harborne JB, Beretz A (1988) Plant flavonoids in biology and medicine II: biochemical, cellular and medicinal properties. Liss, New York
Rice-Evans CA, Miller NJ (1996) Biochem Soc Trans 24:790
Pietta P (2000) J Nat Prod 63:1035
Cao H, Chiang W-X, Pan X-L, Xie X-G, Li T-H (2005) J Mol Struct (Theochem) 719:177
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, New York
Fang YZ, Zheng RL (2002) Theory and application of free radical biology. Science, Beijing
Scott G (1988) Bull Chem Soc Jpn 61:165
Shahidi F (1997) Natural antioxidants: chemistry, health effects and applications. American Oil Chemists Society, Champaign
Thomas CE (1997) In: Packer L, Cadenas E (eds) Handbook of synthetic antioxidants. Dekker, New York
Haslam E (1998) Practical polyphenolics. Cambridge University Press, Cambridge
Garrote G, Cruz JM, Moure A, Dominguez H, Parajo JC (2004) Trends Food Sci Technol 15:191
Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T (2005) Mut Res 579:177
Pietta P, Simonetti P, Maury P (1998) J Agric Food Chem 46:4487
Lee KG, Mitchell AE, Shibamoto T (2000) J Agric Food Chem 48:4817
Lee J, Koo N, Min DB (2004) Comp Rev Food Sci Food Saf 3:21
Sidduraju P (2007) LWT Food Sci Technol 40:982
Trouillas P, Marsal P, Siri D, Lazzaaroni R, Duroux JL (2006) Food Chem 97:679 (and references therein)
Mendoza-Wilson AM, Glossman-Mitnik D (2004) J Mol Struct (Theochem) 681:71
Mendoza-Wilson AM, Glossman-Mitnik D (2006) J Mol Struct (Theochem) 761:97
Espinosa-Garcia J (2004) J Am Chem Soc 126:920
Foti MC, Ingold KU (2003) J Agric Food Chem 51:2758
Hussain HH, Babic G, Durst T, Wright JS, Flueraru M, Chichirau A, Chepelev LL (2003) J Org Chem 68:7023
Sun YM, Zhang HY, Chen DZ, Liu CB (2002) Org Lett 4:2909
Wright JS, Johnson ER, Dilabio GA (2001) J Am Chem Soc 123:1173
Pratt DA, Dilabio GA, Brigati G, Pedulli GF, Valgimigli L (2001) J Am Chem Soc 123:4625
Brigati G, Lucarini M, Mugnaini V, Pedulli GF (2002) J Org Chem 67:4828
Bosque R, Sales J (2003) J Chem Inf Comput Sci 43:637
Bohm BA (1998) Introduction to flavonoids, chap 2. Harwood, Singapore
Robards K, Antolovich M (1997) Analyst 122:11R
Van Acker SABE, Bast A, Van der Vijgh WJF (1998) Structural aspects of antioxidant activity of flavonoids. In: Rice-Evans CA, Packer L (eds) Flavonoids in health and disease. Dekker, New York, p 221
Gunesekaran R, Ubeda A, Alcaraz MJ, Jayaprakasam R, Ramachandran Nair AG (1993) Pharmazie 48:230
Van Acker SABE, De Groot MJ, Van den Berg DJ, Tromp MNJL, Den Kelder GDO, Van der Vijgh WJF, Bast A (1996) Chem Res Toxicol 9:1305
Martins HFP, Leal JP, Fernandez MT, Lobes VHC, Cordeiro MNDS (2004) J Am Chem Soc Mass Spectrum 15:848
Su X-F, Zhang H, Shao J-X, Wu H-Y (2007) J Mol Struct (Theochem) 847:59
Gotoch N, Noguchi N, Tsuchiya J, Morita H, Sakai K, Shimasaki H, Niki E (1996) Free Radical Res 24:123
Noguchi N, Okimoto Y, Tsuchiya J, Cynshi O, Kodama T, Niki E (1997) Arch Biochem Biophys 347:141
Nsangou M, Dhaouadi Z, Jaidane N, Ben Lakhdar Z (2008) J Mol Struct (Theochem) 850:135
Nsangou M, Fifen JJ, Dhaouadi Z, Lahmar S (2008) J Mol Struct (Theochem) 862:53
Balkabassis EG, Chatzopoulou A, Melissas VS, Tsimidou M, Tsolaki M, Vafiadis A (2001) Lipids 36:181
Balkabassis EG, Nemadis E, Tsimidou M (2003) J Am Oil Chem Soc 80:451
Mendoza-Wilson AM, Glossman-Mitnik D (2005) J Mol Struct (Theochem) 716:67
Rice-Evans CA, Miller NJ, Paganga G (1996) Free Radic Biol Med 20:933
Kozlowski D, Marsal P, Steel M, Mokrini R, Duroux JL, Lazzaroni R, Trouillas P (2007) Radiat Res 168:243
Mendoza-Wilson AM, Lardizabal-Gutierrez D, Torres-Moye E, Fuentes-Cobas L, Balandran-Quintana RR, Camacho-Davila A, Quintero-Ramos A, Glossman-Mitnik D (2007) J Mol Struct 871:114
Abraham MH, Grellier PL, Prior DV, Morris JJ, Taylor PJ (1990) J Chem Soc Perkin Trans 2:521
Borges JEM, Borges RS, Alves CN (2004) J Mol Struct (Theochem) 673:93
Parkinson CJ, Mayer PM, Random L (1999) J Chem Soc Perkin Trans 2:2305
Russo N, Toscano M, Uccella N (2000) J Agri Food Chem 48:3232
Andersson MP, Uvdal P (2005) J Phys Chem A 109:2937
Dilabio GA, Pratt DA, LoFaro AD, Wright JS (1999) J Phys Chem A 103:1653
Feng Y, Liu L, Wang JT, Huang H, Guo QX (2003) J Chem Inf Comput Sci 43:2005
Leopoldini M, Prieto Pitarch I, Russo N, Toscano M (2004) J Phys Chem B 108:92
Leopoldini M, Marino T, Russo N, Toscano M (2004) J Phys Chem B 108:4916
Leopoldini M, Marino T, Russo N, Toscano M (2004) Theor Chem Acc 111:210
Shen L, Zhang H-Y, Ji H-F (2008) J Mol Struct (Theochem) 856:119
Scott AP, Randon L (2005) J Phys Chem 100:16502
Parr RG, Donnelly RA, Levy M, Palke WE (1978) J Chem Phys 68:3801
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512
Parr RG, Szentpaly LV, Liu SRG (1999) J Am Chem Soc 121:1922
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham M, Peng CY, Nanayakkara A, Gonzales C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2005) Gaussian 03, Revision D01. Gaussian, Pittsburgh
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senthil kumar, K., Kumaresan, R. A comparative study on the antioxidant properties of bractein and cernuoside by the DFT method. Monatsh Chem 144, 1513–1524 (2013). https://doi.org/10.1007/s00706-013-1024-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-013-1024-5