Abstract
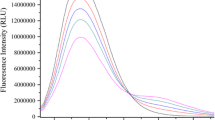
The interaction between a series of rhodanine derivatives with different substituent groups and bovine serum albumin (BSA) was studied using fluorescence quenching spectra, which showed that the type of quenching of BSA by rhodanine derivatives was static in each case. Binding constants, binding site numbers, action distances, and energy transfer efficiencies between donor (BSA) and acceptors (rhodanine derivatives) were calculated and showed that introduction of –NO2 (electron-withdrawing group) is more favorable for rhodanine–BSA interaction than that of –CH3 and –OCH3 (electron-donating groups). Finally, strength of interaction between rhodanine derivatives and BSA was simulated theoretically using quantum chemistry and showed that introduction of groups with small steric hindrance is beneficial to promote the interaction. These investigations are very important to simulate the interaction of small molecules with biomacromolecules and to synthesize drug molecules that interact with protein easily.
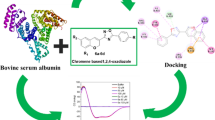
Graphical abstract





Similar content being viewed by others
References
Mcmenamy RH (1997) Albumin structure. Function and uses. Pergamon, Oxford
Chakraborty T, Chakraborty I, Moulik SP, Ghosh S (2009) Langmuir 25:3062
Soares S, Mateus N, Freitas VD (2007) J Agric Food Chem 55:6726
Chakraborty A, Seth D, Setua P, Sarkar N (2006) J Phys Chem B 110:16607
Gull N, Sen P, Khan RH, Din K (2009) Langmuir 25:11686
Tian FF, Jiang FL, Han XL, Xiang C, Ge YS, Li JH, Zhang Y, Li R, Ding XL, Liu Y (2010) J Phys Chem B 114:14842
Saha B, Das G (2009) J Phys Chem C 113:15667
Sahoo D, Bhattacharya P, Chakravorti S (2010) J Phys Chem B 114:10442
Kim IIH, Lee HY, Lee HD, Jung YJ, Tendler SJB, Williams PM, Allen S, Ryu SH, Park JW (2009) Anal Chem 81:3276
Ge SG, Dai P, Yu JH, Zhu YN, Huang JD, Zhang CC, Ge L, Wan FW (2010) Anal Chem 90:1139
Yu JH, Dai P, Ge SG, Zhu YN, Zhang LN, Cheng XL (2009) Spectrochim Acta A 72:17
Yu JH, Dai P, Ge SG, Zhang LN, Tan Y, Li B (2009) Spectrosc Lett 42:42
Yu JH, Ge L, Ping D, Ge SG, Liu SQ (2010) Biosens Bioelectron 25:2065
Haskins-Glusac K, Pinto MR, Tan C, Schanze KS (2004) J Am Chem Soc 126:14964
Rossini JE, Huss AS, Bohnsack JN, Blank DA, Mann KR, Gladfelter WL (2011) J Phys Chem C 115:11
Berndt M, Lorenz M, Enderlein J, Diez S (2010) Nano Lett 10:1497
Someya Y, Yui H (2010) Anal Chem 82:5470
Wang YP, Wei YL, Dong C (2006) J Photochem Photobiol A 177:6
Ross PD, Subramanian S (1981) Biochemistry 20:3096
Förster T (1996) Modern quantum chemistry. Academic, New York
Hu YJ, Yang YO, Dai CM, Liu Y, Xiao XH (2010) Biomacromolecules 11:106
Acknowledgments
This work was financially supported by the Natural Science Research Foundation of China (21207048, 21277058), Technology Development Plan of Shandong Province, China (2011GGB01153), and Natural Science Foundation of Shandong Province, China (ZR2011BQ019, ZR2012BZ002, ZR2011EL029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Li, B., Yin, H. et al. Analysis of the interaction of a new series of rhodanine derivatives with bovine serum albumin by fluorescence quenching. Monatsh Chem 145, 167–173 (2014). https://doi.org/10.1007/s00706-013-0991-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-013-0991-x