Abstract
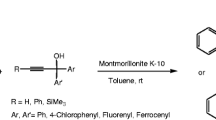
A new solid system based on montmorillonite supported oxone has been developed and used for the oxidation of thiomannoside. The oxidation properties of oxone immobilized on montmorillonite were affected by polarity of the solvent, resulting in the different sulfoxide to sulfone ratios as the products. Toluene was the only solvent favouring sulfone formation over sulfoxide. X-ray crystal structures of the starting compound as well as the corresponding sulfoxide (major epimer Rs) and sulfone are also reported.
Graphical Abstract


Similar content being viewed by others
References
Taylor JG, Li X, Oberthuer M, Zhu W, Kahne DE (2006) J Am Chem Soc 128:15084
Mochizuki T, Kondo Y, Abe H, Taylor CW, Potter BVL, Matsuda A, Shuto S (2006) Org Lett 8:1455
Martin-Lomas M, Khiar N, Garcia S, Koessler J-L, Nieto PM, Rademacher TW (2000) Chem Eur J 6:3608
Lichtenthaler FW, Oberthuer M, Peters S (2001) Eur J Org Chem 20:3849
Crich D, Vinod AU (2003) Org Lett 5:1297
Mannerstedt K, Ekelöf K, Oscarson S (2007) Carbohydr Res 342:631
Kaethip S, Demchenko AV (2011) J Org Chem 76:7388
Moumé-Pymbock M, Crich DJ (2011) Org Chem 77:8905
De Jong A-R, Hagen B, van der Ark V, Overkleeft HS, Codée JDC, van der Marel GA (2011) J Org Chem 77:108
Poláková M, Šesták S, Lattová E, Petruš L, Mucha J, Tvaroška I, Kóňa J (2011) Eur J Med Chem 46:944
Ayers B, Long B, Sim E, Smellie IA, Wilkinson BL, Fairbanks AJ (2009) Carbohydr Res 344:739
Taylor RJK, McAllister GD, Franck RW (2006) Carbohydr Res 341:1298
Leon EI, Martin A, Perez-Martin I, Quintanal LM, Suarez E (2010) Eur J Org Chem 27:5248
Poláková M, Beláňová M, Petruš L, Mikušová K (2010) Carbohydr Res 345:1339
Griffin FK, Paterson DE, Murphy PV, Taylor RJK (2002) Eur J Org Chem 7:1305
Singer M, Lopez M, Bornaghi LF, Innocenti A, Vullo D, Supuran CT, Poulsen S-A (2009) Biorg Med Chem Lett 19:2273
Chen M-Y, Patkar LN, Lin C-C (2004) J Org Chem 69:2884
Chen M-Y, Patkar LN, Chen H-T, Lin C-C (2003) Carbohydr Res 338:1327
Crich D, Cai W, Dai Z (2000) J Org Chem 65:1291
Kakarla R, Dulina RG, Hatzenbuhler NT, Hiu YW, Sofia MJ (1996) J Org Chem 61:8347
Fernandez-Mayoralas A, Marra A, Trumtel M, Veyrieres A, Sinay P (1989) Carbohydr Res 188:81
Gervay J, Flaherty TM, Holmes D (1997) Tetrahedron 53:16355
Belica PS, Franck RW (1998) Tetrahedron Lett 39:8225
Priebe W, Grynkiewicz G (1991) Tetrahedron Lett 32:7353
Agnihotri G, Misra AK (2006) Carbohydr Res 341:275
Morais GR, Humphrey AJ, Falconer RA (2008) Tetrahedron 64:7426
Kropp PJ, Breton GW, Fields JD, Tung JC, Loomis BR (2000) J Am Chem Soc 122:4280
Hirano M, Ueno Y, Morimoto T (1995) Synth Commun 25:3125
Hirano M, Tomaru J, Morimoto T (1991) Bull Chem Soc Jpn 64:3752
Poláková M, unpublished results
Aversa MC, Barattucci A, Bilardo MC, Bonaccorsi P, Giannetto P, Rollin P, Tatiboët A (2005) J Org Chem 70:7389
Acknowledgments
This work was supported by the APVV-51-046505, APVV-0202/10, VEGA-2/0159/12, VEGA 1/0679/11 and VEGA-1/0962/12 grants and the Slovak State Program Project No. 2003SP200280203. Dr. Vladimír Puchart is appreciated for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary data Characterization of the reaction mixture and Figure S1: representative 1H NMR spectrum; X-ray data and Figures S2,S3.
Rights and permissions
About this article
Cite this article
Poláková, M., Jankovič, Ľ., Kucková, L. et al. Application of oxone immobilized on montmorillonite for an efficient oxidation of mannose thioglycoside. Monatsh Chem 144, 969–973 (2013). https://doi.org/10.1007/s00706-013-0964-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-013-0964-0