Abstract
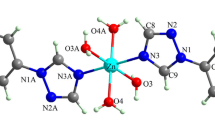
Coordination compounds of first row transition metals from Mn to Zn with clioquinol (5-chloro-7-iodo-8-hydroxyquinoline, CQ) were prepared and characterized by infrared spectroscopy and thermal analysis. The composition of these compounds determined by elemental analysis is [M(CQ)2(H2O)2] for Mn and Zn, [M(CQ)2] for Fe, Co, Ni, and Cu, and NH2(CH3)2[Ni(CQ)3]·DMF·H2O (DMF = N,N-dimethylformamide). X-ray structure analysis revealed that the [Ni(CQ)2] complex is a molecular coordination compound with Ni(II) square-planarly coordinated by nitrogen and oxygen atoms of two trans-arranged bidentate molecules of clioquinol. On the other hand, NH2(CH3)2[Ni(CQ)3]·DMF·H2O is an ionic compound containing three clioquinol molecules coordinated to the central atom in a deformed octahedral geometry thus forming a complex anion. Its negative charge is balanced by the dimethylammonium cation and the structure also contains solvated water and DMF molecules. Long-range interactions and hydrogen bonds in these two complexes were also investigated.
Graphical abstract














Similar content being viewed by others
References
McGrew RE, McGrew MP (1985) Encyclopedia of medical history. McGraw-Hill, New York
Salmon S, Santorelli A (1987) Basic and clinical pharmacology. Appleton & Lange, Norwalk
Zhang CX, Lippard SJ (2003) Curr Opin Chem Biol 7:481
Hollingshead R (1956) Oxine and its derivatives, vol III. Butterworths, London
Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE (2005) Cancer Res 65:3389
Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim YS, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI (2001) Neuron 30:665
LeVine H, Ding Q, Walker JA, Voss RS, Augelli-Szafran CE (2009) Neurosci Lett 465:99
Vaira MD, Bazzicalupi C, Orioli P, Messori L, Bruni B, Zatta P (2004) Inorg Chem 43:3795
Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, DiMonte D, Volitaskis I, Ellerby L, Cherny RA, Bush AI, Andersen JK (2003) Neuron 37:899
Miyashita Y, Ohashi T, Imai A, Amir N, Fujisawa K, Okamoto K (2005) Sci Technol Adv Mater 6:660
Horák M, Papoušek D (1976) Infračervená spektra a struktura molekul. Academia, Praha
Zajcev BE, Andronova NA, Djumaev KM, Smirnov LD (1971) Khim Geterotsikl Soedin 1535
Leon Palomino MI, Zajcev BE, Gashev SB, Nikitin SV, Smirnov LD, Kovalchukova OV (1991) Khim Geterotsikl Soedin 1381
Rospenk M, Leroux N, Zeegers-Huyskens Th (1997) J Mol Spectrosc 183:245
Wagner CC, Calvo S, Torre MH, Baran EJ (2007) J Raman Spectrosc 38:373
Arjunana V, Mohanb S, Ravindranc P, Mythilid CV (2009) Spectrochim Acta A 72:783
González-Baró AC, Baran EJ (1997) Monatsh Chem 128:323
Garcia-Granda S, Gomez-Beltran F (1986) Acta Crystallogr C 42:33
Garcia-Granda S, Beurskens PT, Behm HJJ, Gomez-Beltran F (1987) Acta Crystallogr C 43:39
Kappaun S, Eder S, Sax S, Mereiter K, List EJW, Slugovc CJ (2006) J Mater Chem 16:4389
Gniewek A, Ziolkowski JJ, Lis T (2006) Acta Crystallogr E 62:m1428
Garcia-Granda S, Jansen C, Beurskens PT, Behm HJJ, Gómez-Beltrán F (1988) Acta Crystallogr C 44:176
Diffraction Oxford (2004) Crysalis CCD and crysalis RED. Oxford Diffraction, Oxford
Sheldrick GM (1997) SHELXS97 and SHELXL97. University of Göttingen, Göttingen
Nardelli M (1999) J Appl Crystallogr 32:563
Brandenburg K (2000) DIAMOND (Release 2.1e). Crystal Impact GbR, Bonn
Acknowledgments
This work was supported by the ERDF EU (European Union European regional development fond) grant, under the contract No. ITMS26220120005 and by the internal P.J. Šafárik University grant VVGS PF 27/2011/CH. The authors are very grateful to Prof. Vladimír Zeleňák from P.J. Šafárik University in Košice for the thermal analysis measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Potočňák, I., Vranec, P. Low-dimensional compounds containing bioactive ligands. I: Crystal structure, spectroscopic, and thermal properties of the first row transition metal coordination compounds with clioquinol. Monatsh Chem 143, 217–226 (2012). https://doi.org/10.1007/s00706-011-0678-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0678-0