Abstract
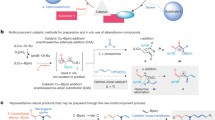
A range of Lewis acid catalysts were used for a series of one-pot multi-component reactions. Of them, silica-supported boron trifluoride (BF3·SiO2) was found to be an effective catalyst for the promotion of the modified Mannich condensation of ketones, aldehydes, and ammonium acetate in 1:2:1 molar ratio to afford 3,5-dialkyl-2,6-diarylpiperidin-4-ones in high yields of 80–92%. Also this simple, easily prepared, and reusable catalyst executed the condensation very effectively in a significantly shorter reaction duration (about 1–4 h) than the conventional method, which requires 1 day or more and involves a tedious work-up procedure. Further, this protocol ensures the stereospecificity; accordingly, all the synthesized piperidones adopted a chair conformation with an equatorial orientation of all substituents at C-2, C-3, C-5, and C-6.
Graphical abstract


Similar content being viewed by others
References
Parthiban P, Aridoss G, Rathika P, Ramkumar V, Kabilan S (2009) Bioorg Med Chem Lett 19:2981
Parthiban P, Balasubramanian S, Aridoss G, Kabilan S (2005) Med Chem Res 14:523
Aridoss G, Parthiban P, Ramachandran R, Prakash M, Kabilan S, Jeong YT (2009) Eur J Med Chem 44:577
Rani M, Parthiban P, Ramachandran R, Kabilan S (2011) Med Chem Res. doi:10.1007/s00044-011-9573-9
El-Subbagh HI, Abu-Zaid SM, Mahran MA, Badria FA, Alobaid AM (2000) J Med Chem 28:2915
Ganellin CR, Spickett RGW (1965) J Med Chem 8:619
Lijinsky W, Taylor HW (1975) Int J Cancer 16:318
Baliah V, Jeyaraman R, Chandrasekaran L (1983) Chem Rev 83:379
Katritzky AR, Fan WJ (1990) J Org Chem 55:3205
Takahata H, Ouchi H, Ichionose M, Nemoto H (2002) Org Lett 4:3459
Honda T, Kimura M (2000) Org Lett 2:3925
Balme G, Bossharth E, Monteiro N (2003) Eur J Org Chem 4101
Wangelin J, Von A, Neumann H, Gordes D, Klaus S, Strubing D, Beller M (2003) Chem Eur J 9:4286
Eilbracht P, Baerfacker L, Buss C, Hollmann C, Kitsos-Rzychon BE, Kranemann CL, Rische T, Roggenbuck R, Schmidt A (1999) Chem Rev 99:3329
Bora U, Saikia A, Boruah RC (2003) Org Lett 5:435
Noller CR, Baliah V (1948) J Am Chem Soc 70:3853
Cramer N, Juretschke J, Laschat S, Baro A, Frey W (2004) Eur J Org Chem 1397
Xu Q, Yang Z, Yin D, Wang J (2006) Front Chem Eng China 3:201
Wang M, Song Z-G, Wan X, Zhao S (2009) Monatsh Chem 140:1205
Bigdeli MA, Nemati F, Mahdavinia GH (2007) Tetrahedron Lett 48:6801
Yadav JS, Reddy BVS, Shankar KS, Premalatha K, Swamy T (2008) Lett Org Chem 5:353
Srinivasan M, Perumal S, Selvaraj S (2005) ARKIVOC xi:201
Dindulkar SD, Parthiban P, Puranik VG, Jeong YT (2011) J Mol Struct 990:44
Klapotke TM, McMonagle F, Spence RR, Winfield JM (2006) J Fluorine Chem 127:1446
Vatsadze SZ, Karinova Yu V, Kovalkina MA, Zyk NV (2000) Chem Heterocycl Comp 36:1185
Acknowledgments
This research work was supported by the Industrial Technology Development Program, which was conducted by the Ministry of Knowledge Economy of the Korean Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dindulkar, S.D., Parthiban, P. & Jeong, Y.T. BF3·SiO2 is a simple and efficient Lewis acid catalyst for the one-pot synthesis of polyfunctionalized piperidin-4-ones. Monatsh Chem 143, 113–118 (2012). https://doi.org/10.1007/s00706-011-0576-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0576-5