Abstract
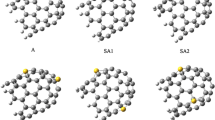
The behavior of the thiocyanate anion (SCN−) adsorbed on the external surface of H-capped (6,0), (7,0), (8,0), and Al-doped (6,0) zigzag single-walled carbon nanotubes was studied by using density functional calculations. Geometry optimizations were carried out at the B3LYP/6-31G* level of theory using the Gaussian 03 suite of programs. We present the nature of the SCN− interaction in selected sites of the nanotubes. Our results show that the pristine carbon nanotubes cannot significantly detect SCN−. The calculated binding energy of the Al-doped (6,0) single-walled carbon nanotubes indicated that SCN− can be adsorbed significantly on the C and Al sites and these nanotubes can therefore be used for SCN− storage. Binding energies corresponding to adsorption of SCN− on the Al site in the Al-doped (6,0) single-walled carbon nanotubes was calculated as −286.38 kJ mol−1. The calculated binding energies for SCN− in N-down orientation are higher than those in S-down orientation for all of the configurations. More efficient binding could not be achieved by increasing the nanotube diameter. We also report the effects of SCN− adsorption on the electronic properties of the nanotubes.
Graphical abstract




Similar content being viewed by others
References
Ijima S (1991) Nature 354:56
Derycke V, Martel R, Appenzeller J, Avouris P (2002) Appl Phys Lett 80:2773
Liu C, Fan YY, Liu M, Cong HT, Cheng HM, Dresselhaus MS (1999) Science 286:1127
Zurek B, Autschbach J (2004) J Am Chem Soc 126:13079
Nojeh A, Lakatos GW, Peng S, Cho K, Pease RFW (2003) Nano Lett 3:1187
Zhou O, Shimoda H, Gao B, Oh SJ, Fleming L, Yue G (2002) Acc Chem Res 35:1045
Zhen Y, Postma HWC, Balents L, Dekker C (1999) Nature 402:273
Baughman RH, Cui C, Zakhidov AA, Iqbal Z, Barisci JN, Spinks GM, Wallace GG, Mazzoldi A, Rossi DD, Rinzler AG, Jaschinski O, Roth S, Kertesz M (1999) Science 284:1340
Gao H, Kong Y, Cui D, Ozkan CS (2003) Nano Lett 3:471
Rawat DS, Calbi MM, Migone AD (2007) J Phys Chem C 111:12980
Zhao J, Buldum A, Han J, Lu JP (2002) Nanotechnology 13:195
Gordillo MC (2007) Phys Rev B 76:115402
Choi YS, Park KA, Kim C, Lee YH (2004) J Am Chem Soc 126:9433
Byl O, Kondratyuk P, Forth ST, Fitzgerald SA, Chen L, Johnson JK, Yatesjr JT (2003) J Am Chem Soc 125:5889
Yang X, Lu Y, Ma Y, Liu Z, Du F, Chen Y (2007) Biotech Lett 29:1775
Gowtham S, Scheicher RH, Ahuja R, Pandey R, Karna S (2007) Phys Rev B 75:033401
Froudakis GE, Schnell M, Muhlhaeser M, Peyerimhoff SD, Andriotis AN, Menon M, Sheetz RM (2003) Phys Rev B 68:115435
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H (2000) Science 287:622
Bekyarova E, Davis M, Burch T, Itkis ME, Zhao B, Sunshine S, Haddon RC (2004) J Phys Chem B 108:19717
Feng X, Irle S, Witek H, Morokuma K, Vidic R, Borguet E (2005) J Am Chem Soc 127:10533
Li J, Lu Y, Ye Q, Cinke M, Han J, Meyyappan M (2003) Nano Lett 3:929
Agnihotri S, Mota JPB, Rostam-Abadi M, Rood MJ (2006) J Phys Chem B 110:7640
Chen RJ, Choi HC, Bangsaruntip S, Yenilmez E, Tang X, Wang Q, Chang YL, Dai H (2004) J Am Chem Soc 126:1563
Chen RJ, Zhang Y, Wang D, Dai H (2001) J Am Chem Soc 123:3838
Kam NWS, Dai H (2005) J Am Chem Soc 127:6021
Peng S, Cho K (2003) Nano Lett 3:513
Fagan SB, Souza Filho AG, Lima JOG, Mendes Filho J, Ferreira OP, Mazali IO, Alves OL, Dresselhaus MS (2004) Nano Lett 4:1285
Zhang Y, Zhang D, Liu C (2006) J Phys Chem B 110:4671
Collins PG, Bradley K, Ishigami M, Zettl A (2000) Science 287:1801
Fitzgerald M, Bowie JH (2004) J Phys Chem A 108:3668
Bierbaum VM, Grabowski JJ, DePuy CH (1984) J Phys Chem 88:1389
Smith D, Spanel P (1995) Mass Spectrom Rev 14:255
Baei MT, SayyedAlang SZ, Soltani AR, Bahari M, Masoodi A (2011) Monatsh Chem 142:1
Peng S, Cho K (2003) Nano Lett 3:513
Kong J, Chapline MG, Dai H (2001) Adv Mater 13:1384
Wei BY, Hsu MC, Su PG, Lin HM, Wu RJ, Lai HJ (2004) Sens Actuators B 101:81
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2003) Gaussian 03, rev. B03. Gaussian, Pittsburgh
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baei, M.T., Soltani, A.R., Torabi, P. et al. Adsorption properties of SCN− on (6,0), (7,0), (8,0), and Al-doped (6,0) zigzag single-walled carbon nanotubes: a density functional study. Monatsh Chem 142, 979–984 (2011). https://doi.org/10.1007/s00706-011-0543-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0543-1