Abstract
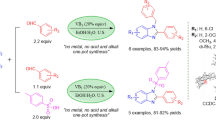
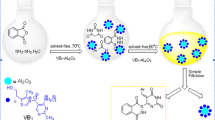
The role of vitamin B1 as a catalyst is investigated for the quinoxaline ring condensation under various mild reaction conditions. The results revealed that the combination of vitamin B1 and ultrasonic irradiation promotes the reaction more efficiently. The salient features of this environmentally benign method are fast conversions, excellent yields for a wide range of substrates, and the use of a low-cost, readily available, nontoxic, and metal-ion-free natural catalyst. The wide range of turnover frequency values (6–400 h−1) shows that the reaction rate is highly dependent on the nature of the functional groups on the aromatic ring of substrates. Moreover, a plausible mechanism for the catalytic action of vitamin B1 has been introduced.
Graphical abstract





Similar content being viewed by others
References
Jordan F, Patel MS (eds) (2004) Thiamine: catalytic mechanisms in normal and disease states. Marcel Dekker, New York
Breslow R (1958) J Am Chem Soc 80:3719
Sheenan JC, Hara T (1974) J Org Chem 39:1196
Shinkai S, Yamashita T, Kusano Y, Manabe O (1980) Tetrahedron Lett 21:2543
Kluger R (1987) Chem Rev 87:863
Scheffold R, Abrecht S, Orlinski R, Ruf H-R, Stamouli P, Tinembart O, Walder L, Weymuth C (1987) Pure Appl Chem 59:363
Dünkelmann P, Jung DK, Nitsche A, Demir AS, Siegert P, Lingen B, Baumann M, Pohl M, Müller M (2002) J Am Chem Soc 124:12084
Malandrinos G, Louloudi M, Hadjiliadis N (2006) Chem Soc Rev 35:684
Mikolajek R, Spiess AC, Büchs J (2007) J Biotech 129:723
Stamatis A, Malandrinos G, Butler IS, Hadjiliadis N, Louloudi M (2007) J Mol Catal A Chem 267:120
Noonan C, Baragwanath L, Connon SJ (2008) Tetrahedron Lett 49:4003
Kluger R, Tittmann K (2008) Chem Rev 108:1797
Pimpim RS, Rubega CCC, de Bravo RVF, Kascheres C (1997) Synth Commun 27:811
Bag S, Vaze VV, Degani MS (2006) J Chem Res 267
Goswami S, Hazra A (2009) Chem Lett 38:484
Lei M, Ma L, Hu L (2009) Tetrahedron Lett 50:6393
Mandhane PG, Joshi RS, Nagargoje DR, Gill CH (2010) Tetrahedron Lett 51:3138
Urleb U (1998) In: Schaumann E (ed) Methods of organic chemistry (Houben-Weyl), Vol. E9b/Part 2 (Hetarenes IV). Thieme, Stuttgart, p 193
Pissot-Soldermann C, Gerspacher M, Furet P, Gaul C, Holzer P, McCarthy C, Radimerski T, Regnier CH, Baffert F, Drueckes P, Tavares GA, Vangrevelinghe E, Blasco F, Ottaviani G, Ossola F, Scesa J, Reetz J (2010) Bioorg Med Chem Lett 20:2609
Ramalingam P, Ganapaty S, Babu Rao C (2010) Bioorg Med Chem Lett 20:406
Tanimori S, Nishimura T, Kirihata M (2009) Bioorg Med Chem Lett 19:4119
Ajani OO, Obafemi CA, Ikpo CO, Ogunniran KO, Nwinyi OC (2009) Chem Heterocycl Compd 45:1370
Weng Q, Wang D, Guo P, Fang L, Hu Y, He Q, Yang B (2008) Eur J Pharmacol 581:262
Villar R, Vicente E, Solano B, Pérez-Silanes S, Aldana I, Maddry JA, Lenaerts AJ, Franzblau SG, Cho S-H, Monge A, Goldman RC (2008) J Antimicrob Chemother 62:547
Carta A, Piras S, Loriga G, Paglietti G (2006) Mini-Rev Med Chem 6:1179
Hassan SY, Khattab SN, Bekhit AA, Amer A (2006) Bioorg Med Chem Lett 16:1753
Perumal RV, Mahesh R (2006) Bioorg Med Chem Lett 16:2769
Jaso A, Zarranz B, Aldana I, Monge A (2005) J Med Chem 48:2019
Brown DJ (2004) Quinoxalines: Supplement II. In: Taylor EC, Wipf P (eds) The chemistry of heterocyclic compounds. Wiley, New Jersey
Huang T-K, Wang R, Shi L, Lu X-X (2008) Catal Commun 9:1143
Srinivas C, Kumar CNSSP, Jayathirtha Rao V, Palaniappan S (2007) J Mol Catal A Chem 265:227
Heravi MM, Bakhtiari K, Tehrani MH, Javadi NM, Oskooie HA (2006) Arkivoc xvi:16
Heravi MM, Tehrani MH, Bakhtiari K, Oskooie HA (2007) Catal Commun 8:1341
Kumar A, Kumar S, Saxena A, De A, Mozumdar S (2008) Catal Commun 9:778
Ajaikumar S, Pandurangan A (2009) Appl Catal A Gen 357:184
More SV, Sastry MNV, Yao C-F (2006) Green Chem 8:91
More SV, Sastry MNV, Wang C-C, Yao C-F (2005) Tetrahedron Lett 46:6345
Bhosale RS, Sarda SR, Ardhapure SS, Jadhav WN, Bhusare SR, Pawar RP (2005) Tetrahedron Lett 46:7183
Cai JJ, Zou JP, Pan XQ, Zhang W (2008) Tetrahedron Lett 49:7386
Mason TJ (1997) Chem Soc Rev 26:443
Cintas P, Luche J-L (1999) Green Chem 1:115
Mason TJ (2007) Ultrason Sonochem 14:476
Mason TJ (2003) Ultrason Sonochem 10:175
Mason TJ, Cintas P (2002) In: Clark J, Macquarrie D (eds) Handbook of green chemistry and technology. Blackwell Science, Oxford, p 372
Aghapoor K, Darabi HR, Mohsenzadeh F, Balavar Y, Daneshyar H (2010) Transit Metal Chem 35:49
Darabi HR, Aghapoor K, Mohsenzadeh F, Taala F, Asadollahnejad N, Badiei A (2009) Catal Lett 133:84
Darabi HR, Tahoori F, Aghapoor K, Taala F, Mohsenzadeh F (2008) J Braz Chem Soc 19:1646
Darabi HR, Mohandessi S, Aghapoor K, Mohsenzadeh F (2007) Catal Commun 8:389
Mohsenzadeh F, Aghapoor K, Darabi HR (2007) J Braz Chem Soc 18:297
Aghapoor K, Darabi HR, Mohsenzadeh F (2005) Z Naturforsch 60b:901
Chakraborti AK, Bhagat S, Rudrawar S (2004) Tetrahedron Lett 45:7641
Sithambaram S, Ding Y, Li W, Shen X, Gaenzlera F, Suib SL (2008) Green Chem 10:1029
Alfonsi K, Colberg J, Dunn PJ, Fevig T, Jennings S, Johnson TA, Kleine HP, Knight C, Nagy MA, Perry DA, Stefaniak M (2008) According to the “solvent selection tool” implemented by the researchers of Pfizer Global Research and Development, CH3OH is considered as a benign and safe solvent in medicinal chemistry. Green Chem 10:31
Nasielski J, Heilporn S, Nasielski-Hinkens R, Geerts-Evrard F (1987) Tetrahedron 43:4329
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghapoor, K., Mohsenzadeh, F., Talebian, S. et al. Vitamin B1 as a metal-ion-free natural catalyst for sustainable quinoxaline ring condensation under sonochemical conditions. Monatsh Chem 142, 619–624 (2011). https://doi.org/10.1007/s00706-011-0487-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0487-5