Abstract
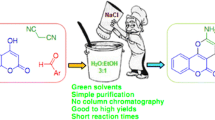
A rapid assembly of the tetracyclic core of marine diterpenoids related to puupehenone and kampanols featuring a Lewis acid catalyzed [4+2] cycloaddition reaction of 1,3,3-trimethyl-2-vinyl-1-cyclohexene and chromone dienophiles is described.
Graphical abstract




Similar content being viewed by others
References
Pindur U, Lutz G, Otto C (1993) Chem Rev 93:741
Loperfido JC (1973) J Org Chem 38:399
Brieger G (1965) Tetrahedron Lett 49:4429
Hollinshed DM, Howell SC, Ley SV, Mahon M, Ratcliffe NM, Worthington PA (1983) J Chem Soc Perkin Trans I 7:1579
Engler TA, Naganathan S, Takusagawa F, Yohannes D (1987) Tetrahedron Lett 28:5267
Engler TA, Sampath U, Velde DV, Takusagawa F (1992) Tetrahedron 48:9399
Engler TA, Naganathan S (1986) Tetrahedron Lett 27:1015
Engler TA, Sampath U, Naganathan S, Velde DV, Takusagawa F, Yohannes D (1989) J Org Chem 54:5712
Knol J, Meetsma A, Feringa BL (1995) Tetrahedron Asymmetr 6:1069
Gorgues A, Stephan D, Belyasmine A, Khanous A, Le Coq A (1990) Tetrahedron 46:2817
Carreno MC, Ruano JLG, Toledo MA (2000) Chem Eur J 6:288
Mayelvaganan T, Hadimani S, Bhat SV (1997) Tetrahedron 53:2185
Hsung RP (1997) J Org Chem 62:7904
Ghosh CK, Bhattacharyya A, Bandyopadhyay C (1984) J Chem Soc Chem Commun 1319
Cremins PJ, Saengchantara T, Wallace TW (1987) Tetrahedron 43:3075
Ravi BN, Perzanowski HP, Ross RA, Erdman TR, Scheuer PJ, Finer J, Clardy J (1979) Pure Appl Chem 51:1893
Amade P, Chevelot L, Perzanowski HP, Scheuer PJ (1983) Helv Chim Acta 66:1672
Kohmoto S, Mc Connell OJ, Wright A, Koehn F, Thompson W, Lui M, Snader KM (1987) J Nat Prod 50:336
Hamann MT, Scheuer PJ, Kelly-Borges M (1993) J Org Chem 58:6565
Nasu SS, Yeung BKS, Hamann MT, Scheuer PJ, Kelly-Borges M, Goins K (1995) J Org Chem 60:7290
Urban S, Capon RJ (1996) J Nat Prod 59:900
Djura P, Stierle DB, Sullivan B, Faulkner DJ, Arnold E, Clardy J (1980) J Org Chem 45:1435
Hamann MT, Scheuer PJ (1991) Tetrahedron Lett 32:5671
Kazlauskas R, Murphy PT, Warren RG, Wells RJ, Blount JF (1978) Aust J Chem 31:2685
Capon RJ (1995) In: Atta-ur-Rahman (ed) Studies in Natural Product Chemistry, vol. 15. Elsevier Science, New York, pp 289–326
Barrero AF, Alvarez-Manzaneda EJ, Chahboun R, Cortes M, Armstrong V (1999) Tetrahedron 55:15181
Bourguet-Kondracki ML, Lacombe F, Guyot M (1999) J Nat Prod 62:1304
El Sayed KA, Bartyzel P, Shen X, Perry TL, Zjawiony JK, Hamann MT (2000) Tetrahedron 56:949
Takamatsu S, Hodges TW, Rajbhandari I, Gerwick WH, Hamann MT, Nagle DG (2003) J Nat Prod 66:605
El Sayed KA, Dunbar DC, Perry TL, Wilkins SP, Hamann T, Greenplate JT, Wideman MA (1997) J Agric Food Chem 45:2735
Singh SB, Zink DL, Williams M, Polishook JD, Sanchez M, Silverman KC, Lingham RB (1998) Bioorg Med Chem Lett 8:2071
Bhar SS, Ramana MMV (2004) J Org Chem 69:8935
Jones GH, Mackenzie JBD, Robertson A, Whalley WB (1949) J Chem Soc 562
Wiley PF (1952) US Patent 2,621,189; Chem Abstr 47:10011h
Chiji H, Aiba T, Izawa M (1978) Agric Biol Chem 42:159
Da Re P (1956) Farmaco, Ed Sci 11:662; Chem Abstr 53:21922d
Badcock GG, Dean FM, Robertson A, Whalley WB (1950) J Chem Soc 903
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (1994) Vogel’s textbook of practical organic chemistry. Longman, Singapore, pp 1193–1194
Schonberg A, Sina A (1950) J Am Chem Soc 72:3396
Pritchard RG, Sheldrake HM, Taylor IZ, Wallace TW (2008) Tetrahedron Lett 49:4156
The Ball-Stick models were generated by using Materials Studio v4.4.0.0030, Accelrys Software Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamble, R.M., Ramana, M.M.V. First Lewis acid catalyzed [4+2] cycloaddition reaction of 1,3,3-trimethyl-2-vinyl-1-cyclohexene with chromones: a new entry to analogues of the puupehenone group of marine diterpenoids and kampanols. Monatsh Chem 142, 501–506 (2011). https://doi.org/10.1007/s00706-011-0480-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0480-z