Abstract
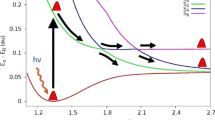
Photoinduced reaction of gas-phase dichlorine molecules on the short wavelength side of the A-band gives a negative value for the asymmetry parameter of Cl (2 P 1/2) fragments, which conflicts with the intrinsic electronic transition mechanism. In this paper, the dissociation process of the dichlorine molecule has been investigated at numerous excitation wavelengths in the range 310–470 nm using the numerical method and the frontier molecular orbital maps are drawn to obtain insight into the character of the relevant molecular orbitals. The possibilities of radial nonadiabatic transition from the C1Πu to the third Ω = 1u (σ u* ← σ g) electronic state are also examined, and found to cause large variations for the angular distribution functions. The wavelength dependence of the beta parameter β 2(Cl*), which is computed from the partial cross-section with the RZD transition mechanism, agrees with the experimental behavior and further justifies the conclusion that the decrease of beta in the Cl + Cl* channel is because of the radial nonadiabatic interaction between the C and 1u(III) excited states and this interaction is a key mechanism decreasing the beta parameter value. At last, the kinetic energy distributions of fragments are obtained in the asymptotic region.
Graphical abstract





Similar content being viewed by others
References
Duhoo T, Pouilly B (1995) J Chem Phys 103:182
DeMille D (2002) Phys Rev Lett 88:67901
Tully JC (1980) Adv Chem Phys 42:63
Atchity GJ, Xantheas SS, Ruedenberg K (1991) J Chem Phys 95:1862
Syage JA, Wessel JA (1988) Appl Spectrosc Rev 24:1
Vitanov NV, Garraway BM (1996) Phys Rev A 53:4288
Kondorskiy A, Nakamura H (2004) J Chem Phys 120:8937
Yang J, Li QS, Zhang SW (2007) Phys Chem Chem Phys 9:466
Lindeman TG, Wiesenfeld (1979) J Chem Phys 70:2882
Zhang D (2010) Acta Physica Polonica A 117:457
Singer SJ, Freed KF, Band YB (1985) Adv Chem Phys 61:1
Hall GE, Houston PL (1989) Annu Rev Phys Chem 40:375
Dixon RN (1986) J Chem Phys 85:1866
Orr-Ewing AJ, Zare RN (1994) Annu Rev Phys Chem 45:315
McCaffrey JG, Kunz H, Schwentner N (1992) J Chem Phys 96:2825
Li L, Lipert RJ, Lobue J, Chupka WA, Colson SD (1988) Chem Phys Lett 151:335
Huang YL, Gordon RJ (1991) J Chem Phys 94:2640
Mulliken RS (1930) Phys Rev 36:1440
Busch GE, Mahoney RT, Morse RI, Wilson KR (1969) J Chem Phys 51:449
Matsumi Y, Kawasaki M, Sato T, Arikawa T (1989) Chem Phys Lett 155:486
Matsumi Y, Tonokura K, Kawasaki M (1992) J Chem Phys 97:1065
Herberg G (1950) Molecular spectra and molecular structure I, spectra of diatomic molecules. Van Nostrand Reinhold, New York, p 319
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities and Huazhong Agricultural University Scientific and Technological Self-innovation Foundation (2009QC016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D. Research on the rupture of the covalent bond of the dichlorine molecule. Monatsh Chem 141, 1279–1285 (2010). https://doi.org/10.1007/s00706-010-0401-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0401-6