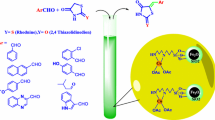
Abstract
Nanocrystalline magnesium oxide with high specific surface area has been used as a novel and efficient catalyst for an improved and rapid synthesis of biologically active 2,4,5-trisubstituted imidazoles, by three-component, one-pot condensation of 1,2-diketones and aryl aldehydes, in excellent yields under solvent-free and conventional heating conditions. The method has several advantages, for example excellent yields, shorter reaction time, and use of a non-toxic and recyclable catalyst.
Graphical abstract




Similar content being viewed by others
References
Lombardino JG, Wiseman EH (1974) J Med Chem 17:1182
Heeres J, Back LJJ, Mostmans JH, Vancutsem J (1979) J Med Chem 22:1003
Mjalli AMM, Sarshar S (1997) US Patent 570082619 (1998) Chem Abstr 128 88918a
Laufer SA, Zimmermann W, Ruff KJ (2004) J Med Chem 47:6311
Grimmett MR (1996) In: Katrizky AR, Rees CW, Scriven EFV (eds) Comprehensive heterocyclic chemistry II, Pergamon Press, Oxford, vol 3, p 77
Kantevari S, Nair CKS, Pardhasaradhi M (2006) J Heterocycl Chem 43:1353
Wolkenberg SE, Wisnoski DD, Leister WH, Wang Y, Zhao Z, Lindsley CW (2004) Org Lett 6:1453
Pozherskii AF, Soldatenkov AT, Kareizky AR (1997) Heterocycles in life and society. Wiley, New York, p 179
Maier T, Schmierer R, Bauer K, Bieringer H, Buerstell H, Sachse B (1989) US Patent 4820335
Liebl R, Handte R, Mildenberger H, Bauer K, Bieringer H (1987) Ger Offen DE 3604042
Wang L, Woods KW, Li Q, Barr KJ, McCroskey RW, Hannick SM, Gherke L, Credo RB, Hui YH, Marsh K, Warner R, Lee JY, Zielinsky-Mozng N, Frost D, Rosenberg SH, Sham HL (2002) J Med Chem 45:1697
Lombardino JG (1972) DE 2155558 [(1973) US 3772441]
Black JW, Durant GJ, Emmett JC, Ganellin CR (1974) Nature 248:65
Ucucu U, Karaburun NG, Iskdag I (2001) Il Farmaco 56:285
Khan MS, Siddiqui SA, Siddiqui MSR, Goswami U, Srinivasan KV, Khan MI (2008) Chem Biol Drug Des 72:197
Chang LL, Sidler KL, Cascieri MA, Laszlo SD, Koch G, Li B, MacCoss M, Mantlo N, O’Keefe S, Pang M, Rolando A, Hagmann WK (2001) Bioorg Med Chem Lett 11:2549
Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Keys JR, Vatter SWL, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR (1994) Nature 372:739
Takle AK, Brown MJB, Davies S, Dean DK, Francis G, Gaiba A, Hird AW, King FD, Lovell PJ, Naylor A, Reith AD, Steadman JG, Wilson DM (2006) Bioorg Med Chem Lett 16:378
Khanna IK, Weier RM, Yu Y, Xu XD, Koszyk FJ, Collins PW, Koboldt CM, Veenhuizen AW, Perkins WE, Casler JJ, Masferrer JL, Zhang YY, Gregory SA, Seibert K, Isakson PC (1997) J Med Chem 40:1634
Lange JHM, Van Stuivenberg HH, Coolen HKAC, Adolfs TJP, McCreary AC, Keizer HG, Wals HC, Veerman W, Borst AJM, de Looff W, Verveer PC, Kruse CG (2005) J Med Chem 48:1823
Gallagher TH, Fier-Thompson SM, Garigipati RS, Sorenson ME, Smietana JM, Lee D, Bender PE, Lee JC, Laydon JT, Griswold DE, Chabot-Fletcher MC, Breton JJ, Adams JL (1995) Bioorg Med Chem Lett 5:1171
Sparks RB, Combs AP (2004) Org Lett 6:2473
Zaman S, Mitsuru K, Abell AD (2005) Org Lett 7:609
Tsuji J, Sakai K, Nemoto H, Nagashima H (1983) J Mol Catal 18:169
Evans DA, Lundy KM (1992) J Am Chem Soc 114:1495
Radziszewski B (1882) Ber Deut Chem Ges 15:1493
Wasserman HH, Long YO, Zhang R, Parr J (2002) Tetrahedron Lett 43:3351
Kamitori Y (2001) J Heterocycl Chem 38:773
Zhang C, Moran EJ, Woiwade TF, Short KM, Mjalli AMM (1996) Tetrahedron Lett 37:751
Lantos I, Zhang WY, Shui Y, Eggleston DS (1993) J Org Chem 58:7092
Bleicher KH, Gerber F, Wuthrich Y, Alanine A, Capretta A (2002) Tetrahedron Lett 43:7687
Balalaie S, Hashemi MM, Akhbari M (2003) Tetrahedron Lett 44:1709
Paone DV, Shaw AW (2008) Tetrahedron Lett 49:6155
Khosropour AR (2008) Ultrason Sonochem 15:659
Wang L, Wang Y, Tian H, Yao Y, Shao J, Liu B (2006) J Fluorine Chem 127:1570
Shen M, Cai C, Yi W (2008) J Fluorine Chem 129:541
Sharma SD, Hazarika P, Konwar D (2008) Tetrahedron Lett 49:2216
Kidwai M, Mothsra P, Bansal V, Somvanshi RK, Ethayathulla AS, Dey S, Singh TP (2007) J Mol Catal A Chem 265:177
Heravi MM, Bakhtiari K, Oskooie HA, Taheri S (2007) J Mol Catal A Chem 263:279
Sharma GVM, Jyothi Y, Sree Lakshmi P (2006) Synth Commun 36:2991
Shaabani A, Rahmati A (2006) J Mol Catal A Chem 249:246
Shaabani A, Rahmati A, Farhangi E, Badri Z (2007) Catal Commun 8:1149
Shelke KF, Sapkal SB, Sonar SS, Madje BR, Shingate BB, Shingare MS (2009) Bull Korean Chem Soc 30:1057
Siddiqui SA, Narkhede UC, Palimkar SS, Daniel T, Lahoti RJ, Srinivasan KV (2005) Tetrahedron 61:3539
Xia M, Lu Y (2007) J Mol Catal A Chem 265:205
Khosropour AR (2008) Can J Chem 86:264
Chary MV, Keerthysri NC, Vupallapati S, Lingaiah N, Kantevari S (2008) Catal Commun 9:2013
Karimi AR, Alimohammadi Z, Amini MM (2009) Mol Diversity. Accessed 29 October 2009
Sangshetti JN, Kokare ND, Kotharkara SA, Shinde DB (2008) J Chem Sci 120:463
Shaabani A, Maleki A, Behnam M (2009) Synth Commun 39:102
Sangshetti JN, Shinde DB, Kokare ND, Kotharkar SA (2008) Monatsh Chem 139:125
Mohamadi AA, Mivechi M, Kefayati H (2008) Monatsh Chem 139:935
Wang L, Cai C (2009) Monatsh Chem 140:541
Jadhave SD, Kokare ND, Jadhave SD (2009) J Heterocycl Chem 45:1461
Sangshetti JN, Kokare ND, Kotharkar SA, Shinde DB (2008) Chin Chem Lett 19:762
Samai S, Nandi GC, Singh P, Singh MS (2009) Tetrahedron 65:10155
Wang XC, Gong HP, Quan ZJ, Li L, Ye HL (2009) Chin Chem Lett 20:44
Gadekar LS, Mane SR, Arbad BR, Katkar SS, Lande MK (2009) Cent Eur J Chem 7:550
Gulkova D, Solcova O, Zdrazil M (2004) Microporous Mesoporous Mater 76:137
Climent MJ, Corma A, Iborra S, Mifsud M (2007) J Catal 247:223
Faghihi-Sani MA, Yamaguchi A (2002) Ceram Int 28:835
Chaim R, Shen ZJ, Nygren MJ (2004) Mater Res 19:2527
Chen D, Jordan EH, Gell M (2008) Scr Mater 59:757
Hattori H (1995) Chem Rev 95:537
Meshkani F, Rezaei M (2010) Powder Technol 199:144
Meshkani F, Rezaei M (2009) Powder Technol 196:85
Acknowledgments
We gratefully acknowledge the financial support from the Research Council of the University of Kashan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safari, J., Khalili, S.D., Rezaei, M. et al. Nanocrystalline magnesium oxide: a novel and efficient catalyst for facile synthesis of 2,4,5-trisubstituted imidazole derivatives. Monatsh Chem 141, 1339–1345 (2010). https://doi.org/10.1007/s00706-010-0397-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0397-y