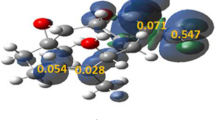
Abstract
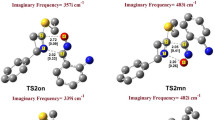
A DFT/B3LYP/6-31G* study was carried out to predict the regio- and stereoselectivities of 1,3-dipolar cycloadditions of C,N-disubstituted aldonitrones to chalcones in terms of FMO theory, DFT-based reactivity indices, and activation energy calculations. The structures of the resultant 2,3,4,5-tetrasubstituted isoxazolidines were determined by means of NMR spectroscopy and X-ray analysis.
Graphical Abstract





Similar content being viewed by others
References
Merino P (2004) In: Padwa A (ed) Science of synthesis, chapter 13 (Nitrones and Analogues). Georg Thieme Verlag, Stuttgart, Germany, 27:511
Torssell KBG (1988) Nitrile oxides, nitrones and nitronates in organic synthesis. VCH, Weinheim, New York
Padwa A, Pearson WH (2002) Synthetic applications of 1, 3-dipolar cycloaddition chemistry towards heterocycles and natural products. Wiley, New York
Chiacchio U, Rescifina A, Iannazzo D, Piperno A, Romeo R, Borrello L, Sciortino MT, Balestrieri E, Macchi B, Mastino A, Romeo G (2007) J Med Chem 50:3747
Iannazzo D, Piperno A, Pistarà V, Rescifina A, Romeo R (2002) Tetrahedron 58:581
Merino P, Revuelta J, Tejero T, Chiacchio U, Rescifina A, Romeo G (2003) Tetrahedron 59:3581
Benchouk W, Mekelleche SM (2008) J Mol Struct Theochem 852:46
Barba C, Carmona D, Garcia JI, Lamata MP, Mayoral JA, Salvatella L, Viguri F (2006) J Org Chem 71:9831
Domingo LR, Aurell MJ, Arno M, Saez JA (2007) J Mol Struct Theochem 811:125
Merino P, Tejero T, Chiacchio U, Romeo G, Rescifina A (2007) Tetrahedron 63:1448
Acharjee N, Das TK, Banerji A, Banerjee M, Prangé T (2010) J Phys Org Chem. doi:10.1002/poc.1690
Banerji A, Maiti KK, Haldar S, Mukhopadhyay C, Banerji J, Prangé T, Neuman A (2000) Monatsh Chem 131:901
Banerji A, Gupta M, Biswas PK, Prangé T, Neuman A (2007) J Heterocycl Chem 44:1045
Banerji A, Biswas PK, Sengupta P, Dasgupta S, Gupta M (2004) Indian J Chem 43B:880
Gilman H (ed) (1941) Org Synth Coll Vol, John Wiley, New York 1:78
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, NewYork
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512
Parr RG, Szentpaly LV, Liu S (1999) J Am Chem Soc 121:1922
Domingo LR, Aurell MJ, Perez P, Contreras R (2002) Tetrahedron 58:4417
Perez P, Domingo LR, Aurell MJ, Contreras R (2003) Tetrahedron 59:3117
Chattaraj PK, Sarkar U, Roy DR (2006) Chem Rev 106:2065
Yang W, Mortier WJ (1986) J Am Chem Soc 108:5708
Chandra AK, Nguyen MT (1997) J Chem Soc Perkin Trans 2:1415
Zhang YL, Yang ZZ (2000) J Mol Struct Theochem 496:139
Koyano K, Suzuki H (1968) Tetrahedron Lett 15:1859
Koyano K, Suzuki H (1969) Bull Soc Chem Japan 42:3306
Taylar TWJ, Sutton LE (1931) J Chem Soc, p 2190
Taylar TWJ, Sutton LE (1933) J Chem Soc, p 63
Foltino K, Lipscomb WN, Jerslev B (1963) Acta Chem Scand 17:2138
Cossio FP, Morao I, Jiao H, Schleyer PVR (1999) J Am Chem Soc 121:6737
Carda M, Portolés R, Murga J, Uriel S, Marco JA, Domingo LR, Zaragoźa RJ, Röper H (2000) J Org Chem 65:7000
Pauling L (1960) The nature of chemical bond, 3rd edn. Cornell University, Ithaca, New York, p 239
Magnuson EC, Pranata J (1998) J Comput Chem 19:1795
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Reed AE, Weinhold F (1983) J Chem Phys 78:4066
Miertus S, Tomasi J (1982) Chem Phys 65:239
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55:117
Barone V, Cossi M (1998) J Phys Chem A 102:1995
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Rev. D.01. Gaussian Inc, Wallingford, CT
Sheldrick GM, Schneider TR (1997) Methods Enzymol 277:319
Acknowledgments
Nivedita Acharjee is thankful to the Council of Scientific and Industrial research, New Delhi (India), for financial support and the University of Calcutta for laboratory and computational facilities. We are thankful to Prof. Manas Banerjee of Burdwan University for some informative discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acharjee, N., Banerji, A. & Prangé, T. DFT study of 1,3-dipolar cycloadditions of C,N-disubstituted aldonitrones to chalcones evidenced by NMR and X-ray analysis. Monatsh Chem 141, 1213–1221 (2010). https://doi.org/10.1007/s00706-010-0393-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0393-2