Abstract
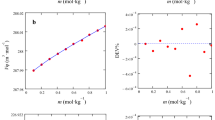
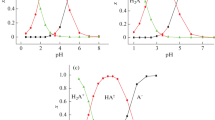
The apparent molar volume of quinic acid and its sodium salt were determined from the density data of aqueous solutions up to molality of 0.4 mol kg−1 and in the temperature range from 293.15 to 328.15 K. The apparent molar volume of sodium quinate comprises the ionic and the associated ion-pair contributions. From the apparent molar volumes of quinic acid and the quinate ion, the molecular contributions to that of quinic acid are derived. At 298.15 K, the limiting apparent molar volume of quinic acid is 119.8 ± 0.5 cm3 mol−1, and that of the quinic ion is 111.6 ± 0.3 cm3 mol−1. Similarly, at 298.15 K, the limiting apparent molar expansibility of sodium quinate is 0.198 ± 0.003 cm3 mol−1 K−1, and that of quinic acid is 0.142 ± 0.003 cm3 mol−1 K−1. From these limiting ionic and molecular apparent molar volumes, the limiting volume change caused by ionization of quinic acid was calculated as −8.2 cm3 mol−1 at 298.15 K. The coefficients of thermal expansion of these solutions were calculated from the density data, and from these the apparent molar expansibilities of quinic acid and its sodium salt were derived.
Graphical abstract






Similar content being viewed by others
References
Barco A, Benetti S, DeRisi C, Marchetti P, Pollini GP, Zanirato V (1997) Tetrahedron Asymmetry 8:3515
Gonzalez C, Carballido M, Castedo L (2003) J Org Chem 68:2248
Marco-Contelles J (2001) Eur J Org Chem 9:1607
Garribba E, Lodyga-Chruscinska E, Sanna D, Micera G (2001) Inorg Chim Acta 322:87
Inomata Y, Haneda T, Howell FS (1999) J Inorg Biochem 76:13
Castillo-Blum SE, Barba-Behrens N (2000) Coord Chem Rev 196:3
Suryaprakash P, Kumar RP, Prakash V (2000) Int J Biol Macromol 27:219
King EJ (1965) Acid-base equilibria. Pergamon, Oxford, p 184
Harned HS, Owen BB (1958) The physical chemistry of electrolytic solutions, 3rd edn. Reinhold, New York, p 370
Wagner W, Pruss A (2002) J Phys Chem Ref Data 31:387
Abell C, Allen FH, Bugg TDH, Doyle MJ, Raithby PR (1988) Acta Cryst C44:1287
Apelblat A (1997) J Mol Liq 73/74:49
Klofutar C, Segatin N (2007) J Solution Chem 36:879
Archer DG, Wang PM (1990) J Phys Chem Ref Data 19:371
Robinson RA, Stokes RH (2002) Electrolyte solutions. Dover, New York, p 468
Redlich O, Meyer DM (1964) Chem Rev 64:221
Millero FJ (1971) Chem Rev 71:147
Schwitzgebel G, Luhrs C, Barthel J (1980) Ber Bunsenges Phys Chem 84:1220
Apelblat A (2002) J Mol Liq 95:99
King EJ (1969) J Phys Chem 73:1220
Klofutar C, Rudan-Tasic D (2006) J Solution Chem 35:395
Kell GS (1975) J Chem Eng Data 20:97
Acknowledgments
We are grateful to C. Klofutar and Rudan Tasič for helpful discussions, and to the Ministry of Higher Education, Science and Technology of the Republic of Slovenia for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poklar Ulrih, N., Šegatin, N. Volumetric properties of aqueous solutions of quinic acid and its sodium salt. Monatsh Chem 141, 1055–1062 (2010). https://doi.org/10.1007/s00706-010-0367-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0367-4