Abstract
An efficient, facile, and mild oxidation of alcohols to the corresponding aldehydes or ketones with potassium peroxodisulfate and 2,2,6,6-tetramethylpiperidinyl-1-oxy in the presence of a catalytic amount of iodobenzene is reported. The oxidation proceeded in a mixed solvent to afford carbonyl compounds in moderate to excellent yields. A possible mechanism for the oxidation is proposed.
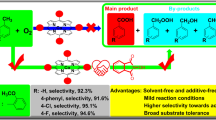
Graphical abstract


Similar content being viewed by others
References
Tojo G, Fernández M (2006) Oxidation of primary alcohols to carboxylic acids. Springer, Berlin
Tojo G, Fernández M (2006) Oxidation of alcohols to aldehydes and ketones. Springer, Berlin
Caron S, Dugger RW, Ruggeri SG, Ragan JA, Ripin DHB (2006) Chem Rev 106:2943
Varvoglis A (1997) Hypervalent iodine in organic chemistry. Academic, London
Stang PJ, Zhdankin VV (1996) Chem Rev 96:1123
Wirth T, Hirt UH (1999) Synthesis 1271
Moriarty RM, Prakash O (1999) Org React 54:273
Zhdankin VV, Stang PJ (2008) Chem Rev 108:5299
Ochiai M (2007) Chem Rec 7:12
Tohma H, Kita Y (2004) Adv Synth Catal 346:111
Richardson RD, Wirth T (2006) Angew Chem Int Ed 45:4402
Ochiai M, Miyamoto K (2008) Eur J Org Chem 4229
Dohi T, Kita Y (2009) Chem Commun 2073
Uyanik M, Ishihara K (2009) Chem Commun 2086
Zhdankin VV (2009) ARKIVOC i:1
Dohi T, Maruyama A, Yoshimura M, Morimoto K, Tohma H, Kita Y (2005) Angew Chem Int Ed 44:6193
Ochiai M, Takeuchi Y, Katayama T, Sueda T, Miyamoto K (2005) J Am Chem Soc 127:12244
Yamamoto Y, Togo H (2006) Synlett 798
Dohi T, Maruyama A, Minamitsuji Y, Takenaga N, Kita Y (2007) Chem Commun 1224
Richardson RD, Page TK, Altermann S, Paradine SM, French AN, Wirth T (2007) Synlett 538
Akiike J, Yamamoto Y, Togo H (2007) Synlett 2168
Yamamoto Y, Kawano Y, Toy PH, Togo H (2007) Tetrahedron 63:4680
Richardson RD, Desaize M, Wirth T (2007) Chem Eur J 13:6745
Moroda A, Togo H (2008) Synthesis 1257
Dohi T, Maruyama A, Takenage N, Senami K, Minamitsuji Y, Fujioka H, Caemmerer S, Kita Y (2008) Angew Chem Int Ed 47:3787
Quideau S, Lyvinec G, Marguerit M, Bathany K, Beaudenon AO, Buffeteau T, Cavagnat D, Chenede A (2009) Angew Chem Int Ed 48:4605
Miyamoto K, Sei Y, Yamaguchi K, Ochiai M (2009) J Am Chem Soc 131:1382
Uyanik M, Yasui T, Ishihara K (2009) Bioorg Med Chem Lett. doi:10.1016/j.bmcl.2009.03.148
Thottumkara AP, Bowsher MS, Vinod TK (2005) Org Lett 7:2933
Schulze A, Giannis A (2006) Synthesis 257
Lei Z, Yan P, Yang Y (2007) Catal Lett 118:69
Yakura T, Konishi T (2007) Synlett 765
Yakura T, Tian Y, Yamauchi Y, Omoto M, Konishi T (2009) Chem Pharm Bull 57:252
Yakura T, Omoto M (2009) Chem Pharm Bull 57:643
Uyanik M, Akakura M, Ishihara K (2009) J Am Chem Soc 131:251
Ojha LR, Kudugunti S, Maddukuri PP, Kommareddy A, Gunna MR, Dokuparthi P, Gottam HB, Botha KK, Parapati DR, Vinod TK (2009) Synlett 117
Sheng J, Li X, Tang M, Gao B, Huang G (2007) Synthesis 1165
Dohi T, Minamitsuji Y, Maruyama A, Hirose S, Kita Y (2008) Org Lett 10:3559
Page PCB, Appleby LF, Buckley BR, Allin SM, Mckenzie MJ (2007) Synlett 1565
Liu H, Tan C-H (2007) Tetrahedron Lett 48:8220
Mu R, Liu Z, Yang Z, Liu Z, Wu L, Liu Z-L (2005) Adv Synth Catal 347:1333
Yusubov MS, Zagulyaeva AA, Zhdankin VV (2009) Chem Eur J. doi:10.1002/chem.200901953
Minamitsuji Y, Kato D, Fujioka H, Dohi T, Kita Y (2009) Aust J Chem 62:648
Herrerías CI, Zhang TY, Li C-J (2006) Tetrahedron Lett 47:13
Sheldon RA, Arends IWCE (2004) Adv Synth Catal 346:1051
Ishii Y, Sakaguchi S (2006) Catal Today 117:105
Sheldon RA, Arends IWCE (2006) J Mol Catal A Chem 251:200
Ishii Y, Sakaguchi S, Iwahama T (2001) Adv Synth Catal 343:393
De Mico A, Margarita R, Parlanti L, Vescovi A, Piancatelli G (1997) J Org Chem 62:6974
Liu L, Ji L, Wei Y (2008) Monatsh Chem 139:901
Wei Y, Cai M, Lu C (2003) Catal Lett 90:81
Liu L, Ma J, Wei Y (2008) J Mol Catal A Chem 291:1
Liu L, Ji L, Wei Y (2008) Catal Commun 9:1379
Pouchert CJ (1985) The Aldrich Library of FT-IR Spectra. Aldrich Chemical Co., Milwaukee: (a) 2,104A; (b) 2,112D; (c) 2,111B; (d) 2,110C; (e) 2,8B; (f) 1,1264D; (g) 2,102A; (h) 1,1066C; (i) 1,432C; (j) 2,581B; (k) 1,468C; (l) 1,410B; (m) 1,469D
Pouchert CJ, Behnke J (1992) The Aldrich Library of 13C and 1H FT NMR Spectra. Aldrich Chemical Co., Milwaukee: (a) 2,932B; (b) 2,945A; (c) 2,941A; (d) 2,940B; (e) 2,802A; (f) 2,884C; (g) 2,926C; (h) 2,230A; (i) 1,669A; (j) 3,17A; (k) 1,731C; (l) 1,638C; (m) 1,361B
Hossain MD, Kitamura T (2006) Bull Chem Soc Jpn 79:142
Acknowledgments
We are grateful to Nanjing University of Science and Technology for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, C., Ji, L. & Wei, Y. Catalytic hypervalent iodine oxidation of alcohols to corresponding aldehydes or ketones using 2,2,6,6-tetramethylpiperidinyl-1-oxy and potassium peroxodisulfate. Monatsh Chem 141, 327–331 (2010). https://doi.org/10.1007/s00706-010-0260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0260-1