Abstract
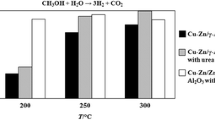
Impregnated Cu–Zn over Al2O3 exhibits high activity with the use of a lower amount of active metal relative to conventional co-precipitation catalysts. The activity of the catalyst could be enhanced by addition of urea to the metal salt solution during impregnation. The H2 yield from Cu–Zn catalysts with urea is 42%, while the H2 yield from catalyst without urea is only 28% in a continuous system at 250 °C and 1.2 atm. The H2 yield of the catalyst with urea in this study could compete with that of commercial catalysts. The role of urea in the Cu–Zn catalysts was investigated. X-ray diffraction (XRD) analysis of the catalysts shows that the crystal size of CuO could be reduced by the addition of urea. The XRD diffractogram of the catalyst prior to calcination also shows the formation of NH4NO3, which could aid in dissociation of metal clusters. Scanning electron microscopy (SEM) images of catalysts show the size of Cu–Zn compound clusters and also their dispersion over the Al2O3 surface on the impregnated catalysts. The addition of urea could also yield smaller Cu–Zn compound clusters and better dispersion compared with the impregnated catalyst without urea. Such impregnated Cu–Zn catalysts with urea could be alternative novel catalysts for methanol steam reforming.
Graphical abstract








Similar content being viewed by others
References
Alessandra FL, Elisabete MA (2006) J Power Sources 159:667
Balcerowiak W, Perkowski I (1987) J Therm Anal 32:1777
Basile A, Parmaliana A, Tosti S, Iulianelli A, Gallucci F, Espro C, Spooren J (2008) Catal Today 137:17
Campos Skrobot FC, Rizzo Domingues RCP, Fernandes Machado NRC, Cantão MP (2008) J Power Sources 183:713
Costantino U, Marmottini F, Sisani M, Montanari T, Ramis G, Busca G, Turco M, Bagnasco G (2005) Solid State Ionics 176:2917
Koga H, Fukahori S, Kitaoka T, Tomoda A, Suzuki R, Wariishi H (2006) Appl Catal A 309:263
Morozov IV, Fedorova AA, Knotko AV, Valedinskaja OR, Kemnitz E (2004) Mendeleev Commun 14:138
Murcia-Mascaros S, Navarro RM, Gomez-Sainero L, Costantino U, Nocchettiy M, Fierro JLG (2001) J Catal 198:338
Papavasiliou J, Avgouropoulos G, Ioannides T (2007) Appl Catal B 69:226
Patel S, Pant KK (2006) J Power Sources 159:139
Patel S, Pant KK (2007) Chem Eng Sci 62:5436
Pingali KC, Deng S, Rockstraw DA (2008) Res Lett Nanotechnol. doi:10.1155/2008/756843
Shishido T, Yamamoto Y, Morioka H, Takaki K, Takehira K (2004) Appl Catal A 263:249
Shishido T, Yamamoto Y, Morioka H, Takehira K (2007) J Mol Catal A: Chem 268:185
Turco M, Bagnasco G, Costantino U, Marmottini F, Montanari T, Ramis G, Busca G (2004) J Catal 228:43
Turco M, Bagnasco G, Costantino U, Marmottini F, Montanari T, Ramis G, Busca G (2004) J Catal 228:56
Xu J, Yeung CMY, Ni J, Meunier F, Acerbi N, Fowles M, Tsang SC (2008) Appl Catal A 345:119
Yao CZ, Wang LC, Liu YM, Wu GS, Cao Y, Dai WL, He HY, Fan KN (2006) Appl Catal A 297:151
Zhou L, Guo Y, Zhang Q, Yagi M, Hatakeyama J, Li H, Chen J, Sakurai M, Kameyama H (2008) Appl Catal A 347:200
Acknowledgments
The authors gratefully acknowledge Dr. Sumittra Charojrochkul from the National Metal and Materials Technology Center Thailand for providing experimental facilities. We also thank Dr. Paul Jerus for commercial catalyst and Mr. Paul Vincent Neilson for English proof-reading. Finally, we would like to thank the Thailand Research Fund (TRF) for financial support (grant MRG4780120).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henpraserttae, S., Limthongkul, P. & Toochinda, P. The role of urea in Cu–Zn–Al catalysts for methanol steam reforming. Monatsh Chem 141, 269–277 (2010). https://doi.org/10.1007/s00706-010-0256-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0256-x