Abstract
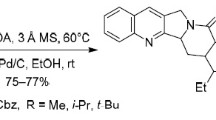
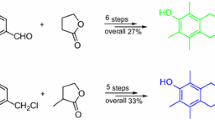
A concise formal synthesis of camptothecin is described. The key pyrido-lactone (DE ring) was prepared effectively starting from 2-chloronicotinic acid via lithiation, reduction, and hydrolysis in 29% overall yield.
Graphical abstract





Similar content being viewed by others
References
Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA (1966) J Am Chem Soc 88:3888
Hsiang YH, Hertzberg R, Hecht S, Liu LF (1985) J Biol Chem 260:14873
Holm C, Covey JM, Kerrigan D, Pommier Y (1989) Cancer Res 49:6365
Kingsbury WD, Boehm JC, Jakas DR, Holden KG, Hecht SM, Gallagher G, Caranfa MJ, McCabe FL, Faucette LF, Johnson RK, Hertzberg RP (1991) J Med Chem 34:98
Negoro S, Fukuoka M, Masuda N, Takada M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Niitani H, Taguchi TJ (1991) J Natl Cancer Inst 83:1164
Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K (1991) Cancer Res 51:4187
Priel E, Showalter SD, Blair DG (1991) AIDS Res Hum Retroviruses 7:65
Cragg GM, Newman DJ (2004) J Nat Prod 67:232
Stork G, Schultz AG (1971) J Am Chem Soc 93:4074
Corey EJ, Crouse DN, Anderson JE (1975) J Org Chem 40:2140
Plattner JJ, Gless RD, Cooper GK, Rapoport H (1974) J Org Chem 39:303
Wani MC, Ronman PE, Lindley JT, Wall ME (1980) J Med Chem 23:554
Hutchinson CR (1981) Tetrahedron 37:1047
Ciufolini MA, Roschangar F (1997) Tetrahedron 53:11049
Wall ME, Wani MC (1998) Alkaloids 50:509
Tagami K, Nakazawa N, Sano S, Nagao Y (2000) Heterocycles 53:771
Comins DL, Nolan JM (2001) Org Lett 3:4255
Du W (2003) Tetrahedron 59:8649
Yu J, Depue J, Kronenthal D (2004) Tetrahedron Lett 45:7247
Twin H, Batey RA (2004) Org Lett 6:4913
Thomas OP, Dumas C, Zaparucha A, Husson HP (2004) Eur J Org Chem 5:1128
Anderson RJ, Raolji GB, Kanazawa A, Greene AE (2005) Org Lett 7:2989
Brunin T, Hénichart JP, Rigo B (2005) Tetrahedron 61:7916
Rahier NJ, Cheng K, Gao R, Eisenhauer BM, Hecht SM (2005) Org Lett 7:835
Peters R, Althaus M, Nagy AL (2006) Org Biomol Chem 4:498
Li QY, Zu YG, Shi RZ, Yao LP (2006) Curr Med Chem 13:2021
Elban MA, Sun W, Eisenhauer BM, Gao R, Hecht SM (2006) Org Lett 8:3513
Dai W, Petersen JL, Wang KK (2006) Org Lett 8:4665
Xiao X, Antony S, Pommier Y, Cushman M (2006) J Med Chem 49:1408
Hiroya K, Kawamoto K, Sakamoto T (2006) Synlett 2636
Tang CJ, Babjak M, Anderson RJ, Greene AE, Kanazawa A (2006) Org Biomol Chem 4:3757
Chavan SP, Pathak AB, Kalkote UR (2007) Tetrahedron Lett 48:6561
Zhou HB, Liu GS, Yao ZJ (2007) Org Lett 9:2003
Zhou HB, Liu GS, Yao ZJ (2007) J Org Chem 72:6270
Liu GS, Dong QL, Yao YS, Yao ZJ (2008) Org Lett 10:5393
Chavan SP, Dhawane AN, Kalkote UR (2008) Synlett 2781
Cheng KJ, Rahier NJ, Eisenhauer BM, Gao R, Thomas SJ, Hecht SM (2005) J Am Chem Soc 127:838
Brunin T, Legentil L, Hénichart J-P, Rigo B (2006) Tetrahedron 62:3959
Cinelli MA, Morrell A, Dexheimer TS, Scher ES, Pommier Y, Cushman M (2008) J Med Chem 51:4609
Zhang LP, Bao Y, Kuang YY, Chen FE (2008) Helv Chim Acta 91:2057
Pin F, Comesse S, Sanselme M, Daïch A (2008) J Org Chem 73:1975
Boisse T, Gavara L, Hénichart J-P, Rigo B, Gautret P (2009) Tetrahedron 65:2455
Curran DP, Liu H (1991) J Am Chem Soc 113:2127
Wall ME, Wani MC, Natschke SM, Nicholas AW (1986) J Med Chem 29:1553
Ciufolini MA, Roschangar F (1990) Angew Chem Int Ed 35:1692
Chavan SP, Venkatraman MS (1998) Tetrahedron Lett 39:6745
Fortunak JMD, Mastrocola AR, Mellinger M, Sisti NJ, Wood JL, Zhuang ZP (1996) Tetrahedron Lett 37:5679
Boger DL, Hong J (1998) J Am Chem Soc 120:1218
Comins DL, Hang H, Saha JK, Jianhua G (1994) J Org Chem 59:5120
Lazaar J, Hoarau C, Mongin F, Trécourt F, Godard A, Quéguiner G, Marsais F (2005) Tetrahedron Lett 46:3811
Mongin F, Trécourt F, Quéguiner G (1999) Tetrahedron Lett 40:5483
Godard A, Marsais F, Plé N, Trécourt F, Turck A, Quéguiner G (1995) Heterocycles 40:1055
Quéguiner G, Marsais F, Snieckus V, Epsztajn J (1991) Adv Heterocycl Chem 52:187
Trécourt F, Gervais B, Mongin O, Le Gal C, Mongin F, Quéguiner G (1998) J Org Chem 63:2892
Pasquinet E, Rocca P, Marsais F, Godard A, Quéguiner G (1998) Tetrahedron 54:8771
Mortier J, Moyroud J, Bennetau B, Cain PA (1994) J Org Chem 59:4042
Bennetau B, Mortier J, Moyroud J, Guesnet JL (1995) J Chem Soc Perkin Trans 1 1265
Acknowledgments
We gratefully acknowledge financial support from the Program for New Century Excellent Talents in University (NCET) and National Natural Science Foundation of China. We also thank the Laboratory of Organic Functional Molecules, Sino-French Institute of ECNU for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, S., Luo, Y., Liu, H. et al. Synthesis of the DE synthon of racemic camptothecin. Monatsh Chem 141, 245–249 (2010). https://doi.org/10.1007/s00706-009-0245-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-009-0245-0