Abstract
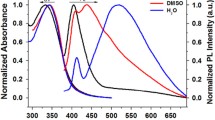
Novel bilirubin analogs with two nonionic, water-solubilizing polyethyleneglycol (PEG) β-substituents of varying chain length on the lactam ring of each dipyrrinone were synthesized. In contrast to bilirubin, which is insoluble in CH3OH and in H2O at pH 7 but somewhat soluble in CHCl3 (~1 mM), the PEGylated rubins are soluble in all three solvents, with H2O solubility increasing with increasing number of ethyleneglycol units in the PEG chain(s). Vapor pressure osmometry indicates that, like bilirubin, they are monomeric in CHCl3 and in CH3OH. Nuclear magnetic resonance (NMR) studies indicate that their most stable structure in these solvents and in H2O has the 4Z,15Z configuration that is bent into a ridge-tile shape with the pigment’s dipyrrinones engaged in intramolecular hydrogen bonding to the propionic acid carboxyl groups. Aqueous pK a values for the intramolecularly hydrogen-bonded carboxyl groups of these compounds, determined by vacuum-assisted multiplexed capillary electrophoresis in H2O–CH3OH mixtures followed by Yesuda-Shedlovsky extrapolations to pure H2O, were found to be 4.9, as previously determined by NMR titrations for mesobilirubin-XIIIα.
Graphical abstract






Similar content being viewed by others
References
Chowdhury JR, Wolkoff AW, Chowdhury NR, Arias IM (2001) Hereditary jaundice and disorders of bilirubin metabolism. In: Scriver CF, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, chap 125, McGraw-Hill, New York, pp 3063–3101
Lightner DA, McDonagh AF (1984) Acc Chem Res 17:417
McDonagh AF, Lightner DA (1985) Pediatrics 75:443
McDonagh AF (1979) Bile pigments: Bilatrienes and 5,15-biladienes. In: Dolphin D (ed) The porphyrins, chap 6, vol VI, Academic Press, New York
Schmid R, McDonagh AF (1978) Hyperbilirubinemia. In: Stanbury JB, Wyngarden JB, Fredrickson DS (eds) Basis of inherited disease, 4th edn. McGraw-Hill, New York
Falk H (1989) The chemistry of linear oligopyrroles and bile pigments. Springer, Wien
Person RV, Peterson BR, Lightner DA (1994) J Am Chem Soc 116:42
Bonnett R, Davies JE, Hursthouse NB, Sheldrick GM (1978) Proc R Soc London Ser B 202:249
LeBas G, Allegret A, Mauguen Y, DeRango C, Bailly M (1980) Acta Crystallogr Sect B 36:3007
Becker W, Sheldrick WS (1978) Acta Crystallogr Sect B 34:1298
Sheldrick WS (1983) Israel J Chem 23:55
Sheldrick WS (1976) J Chem Soc Perkin 2, 1457
Brodersen R (1986) Aqueous solubility, albumin binding, and tissue distribution of bilirubin. In: Ostrow JD (ed) Bile pigments and jaundice. Marcel Dekker, New York, pp 157–181
Brodersen R (1979) J Biol Chem 254:2364
Ghosh B, Lightner DA, McDonagh AF (2004) Monatsh Chem 135:1189
Xie M, Holmes DL, Lightner DA (1993) Tetrahedron 49:9235
Brower JO, Lightner DA, McDonagh AF (2000) Tetrahedron 56:7869
Boiadjiev SE, Watters K, Lai B, Wolf S, Welch W, McDonagh AF, Lightner DA (2004) Biochemistry 43:15617
Dey SK, Lightner DA (2008) J Org Chem 73:2704
Dey SK, Lightner DA (2009) Monatsh Chem 140:161
Boiadjiev SE, Lightner DA (1994) Synlett 777
Boiadjiev SE, Lightner DA (2006) Org Prep Proced Int 38:347
Brower JO, Lightner DA, McDonagh AF (2001) Tetrahedron 57:7813
Brower JO, Lightner DA (2001) Monatsh Chem 132:1527
Brower JO, Huggins MT, Boiadjiev SE, Lightner DA (2000) Monatsh Chem 131:1047
Huggins MT, Lightner DA (2000) J Org Chem 65:6001
Boiadjiev SE, Anstine DT, Lightner DA (1995) J Am Chem Soc 117:8727
Boiadjiev SE, Anstine DT, Maverick E, Lightner DA (1995) Tetrahedron Asymmetry 6:2253
Nogales DF, Ma J-S, Lightner DA (1993) Tetrahedron 49:2361
Navon G, Frank S, Kaplan D (1984) J Chem Soc Perkin 2, 1145
Tipton AK, Lightner DA, McDonagh AF (2001) J Org Chem 66:1832
Ghosh B, Lightner DA (2003) J Heterocycl Chem 40:1113
Kasha M, El-Bayoumi MA, Rhodes W (1961) J Chim Phys Chim Biol 58:916
Falk H, Vormayr G, Margulies L, Metz S, Mazur Y (1986) Monatsh Chem 117:849
Falk H, Grubmayr K, Höllbacher G, Hofer O, Leodolter A, Neufingerl F, Ribó JM (1977) Monatsh Chem 108:1113
Lightner DA, Gawroński JK, Wijekoon WMD (1987) J Am Chem Soc 109:6354
Lightner DA, Wijekoon WMD, Zhang MH (1988) J Biol Chem 263:16669
Pu Y-M, McDonagh AF, Lightner DA (1993) J Am Chem Soc 115:377
Shalaeva M, Kenseth J, Lombardo F, Bastin A (2008) J Pharm Sci 97:2581
Yesuda M (1959) Bull Chem Soc Jpn 32:429
Shedlovsky T (1962) In: Pesce B (ed) Electrolytes. Pergamon, New York
Avdeef A, Box KJ, Comer JEA, Gilges M, Hadley M, Hibbert C, Patterson W, Tam KY (1999) J Pharm Biomed Anal 20:632
Acknowledgments
We thank the US National Institutes of Health (HD 17779) for generous support of this work and the National Science Foundation (CHE-0521191) for providing funding for acquisition of a 400-MHz NMR spectrometer and upgrade of our 500-MHz NMR. We thank Prof. A.F. McDonagh (Univ. California, San Francisco) for running the HPLCs of 1–4. We also thank Prof. T.W. Bell for use of the VPO apparatus and Dr. Stephen Spain for assistance with the water-suppression NMR experiments. We thank Ms. Jolanta Plewa and Dr. Christopher Welch of Merck Research Labs for the VAMCE measurements of pK a.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dey, S.K., Lightner, D.A. Lipid- and water-soluble bilirubins. Monatsh Chem 141, 101–109 (2010). https://doi.org/10.1007/s00706-009-0232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-009-0232-5