Abstract
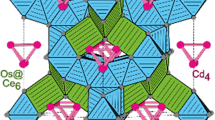
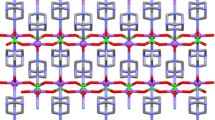
The palladium-rich cadmium compounds La6Pd13Cd4 and Ce6Pd13Cd4 were synthesized by induction melting the elements in sealed tantalum ampoules and subsequent annealing. They were characterized by X-ray powder and single-crystal diffraction: Na16Ba6N type, \(Im\overline{3} m\), a = 988.12(9) pm, wR2 = 0.0463, 225 F 2 values, and 12 variables for La6Pd13Cd4, and a = 982.1(2) pm, wR2 = 0.0521, 215 F 2 values, and 12 variables for Ce6Pd13Cd4. The striking structural motifs are palladium-centred La6 and Ce6 octahedra, which are packed in a bcc fashion. Further palladium and cadmium atoms built up three-dimensional [Pd3Cd] networks in which the La6Pd and Ce6Pd octahedra are embedded. Chemical bonding analyses show that the dominant interaction occurs within the palladium-centred RE 6 octahedra, while weaker bonding exists between them.
Graphical abstract





Similar content being viewed by others
References
Rodewald UC, Chevalier B, Pöttgen R (2007) J Solid State Chem 180:1720
Iandelli A (1992) J Alloys Compd 182:87
Horechyy AI, Pavlyuk VV, Bodak OI (1999) Pol J Chem 73:1681
Pavlyuk VV, Horechyj AI, Kevorkov DG, Dmytriv GS, Bodak OI, Koziol JJ, Ciesielski W, Kapuśniak J (2000) J Alloys Compd 296:276
Mishra R, Pöttgen R, Hoffmann R-D, Kaczorowski D, Piotrowski H, Mayer P, Rosenhahn C, Mosel BD (2001) Z Anorg Allg Chem 627:1283
Lukachuk M, Pöttgen R (2003) Z Kristallogr 218:767
Schappacher FM, Hermes W, Pöttgen R (2009) J Solid State Chem 182:265
Doğan A, Rayaprol S, Pöttgen R (2007) J Phys Condens Matter 19:076213
Schappacher FM, Pöttgen R (2008) Monatsh Chem 139:1137
Schappacher FM, Rodewald UC, Pöttgen R (2008) Z Naturforsch 63B:1127
Tappe F, Pöttgen R (2009) Z Naturforsch 64B:184
Tappe F, Hermes W, Eul M, Pöttgen R (2009) Intermetallics 17:1035
Doğan A, Hoffmann R-D, Pöttgen R (2007) Z Anorg Allg Chem 633:219
Snyder GJ, Simon A (1994) Angew Chem 106:713
Sheldrick GM (1997) Shelxl-97: program for crystal structure refinement. University of Göttingen, Göttingen
Emsley J (1999) The elements. Oxford University Press, Oxford
Dwight AE, Downey JW, Conner RA Jr (1961) Acta Crystallogr 14:75
Yuan-Tao N, Xin-Ming Z, Yun Z, Nian-Yi C, Hua X, Jian-Zhong Z (1989) J Less Common Met 147:167
Rayaprol S, Doğan A, Pöttgen R (2006) J Phys Condens Matter 18:5473
Zaremba R, Rodewald UC, Pöttgen R (2007) Z Naturforsch 62B:1574
Tuncel S, Chevalier B, Pöttgen R (2008) Z Naturforsch 63B:600
Pöttgen R, Fugmann A, Hoffmann R-D, Rodewald UC, Niepmann D (2000) Z Naturforsch 55B:155
Donohue J (1974) The structures of the elements. Wiley, New York
Harris IR, Raynor GV (1965) J Less Common Met 9:263
Hutchens RD, Rao VUS, Greedan JE, Wallace WE, Craig RS (1971) J Appl Phys 42:1293
Pöttgen R, Gulden T, Simon A (1999) GIT Labor Fachzeitschrift 43:133
Kußmann D, Hoffmann R-D, Pöttgen R (1998) Z Anorg Allg Chem 624:1727
Yvon K, Jeitschko W, Parthé E (1977) J Appl Crystallogr 10:73
Williams AR, Kübler J, Gelatt CD (1979) Phys Rev B 19:6094
Eyert V (2007) The augmented spherical wave method: a comprehensive treatment (Lect Notes Phys 719). Springer, Berlin
Hohenberg P, Kohn W (1964) Phys Rev 136:B864
Kohn W, Sham LJ (1965) Phys Rev 140:A1133
Vosko SH, Wilk L, Nusair M (1980) Can J Phys 58:1200
Hoffmann R (1987) Angew Chem Int Ed 26:846
Acknowledgments
This work was financially supported by the Deutsche Forschungsgemeinschaft. We are thankful to Dipl.-Ing. U. Ch. Rodewald for the intensity data collections. The computations benefited from the M3PEC-Mesocentre/University Bordeaux 1 facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tappe, F., Matar, S.F. & Pöttgen, R. La6Pd13Cd4 and Ce6Pd13Cd4 with palladium-centred rare earth octahedra: synthesis, structure, and chemical bonding. Monatsh Chem 141, 1–6 (2010). https://doi.org/10.1007/s00706-009-0224-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-009-0224-5