Abstract
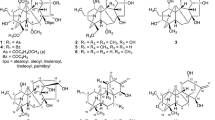
Salicylic acid (SA) and methyl jasmonate (MJ) are important plant signal molecules to cause systemic acquired resistance (SAR), while it’s reported that they also have wide spectrum antitumor activities. Benzothiadiazole-7-carboxylates are plant activators which can cause SAR just like SA and MJ. To investigate whether the benzothiadiazole-7-carboxylate family is endowed with anticancer activities, several benzothiadiazole-7-carboxylate derivatives are synthesized and their inhibition to P388 murine leukemia cell and A549 human lung cancer cell compared with MJ are evaluated. The data indicated that benzo-1,2,3-thiadiazole-7-carboxylic acid 2-benzoyloxyethyl ester has a higher inhibition ability to the cancer cell P388 and A549, compared with MJ.
Similar content being viewed by others
References
JE Alexander Yalpanl Nasser (1992) Cell 70 879 Occurrence Handle10.1016/0092-8674(92)90239-9
K Lidija (1999) Blood 93 2386
H Grundlach MJ Müller TM Kutchan (1992) Proc Natl Acad Sci 89 2389 Occurrence Handle10.1073/pnas.89.6.2389
E Demole E Lederer D Mercier (1962) Helv Chim Acta 45 675 Occurrence Handle10.1002/hlca.19620450233 Occurrence Handle1:CAS:528:DyaF38XktFSmsbk%3D
G Haider TV Schrader M Fußlein S Blechert TM Kutchan (2000) Biol Chem 381 741 Occurrence Handle10.1515/BC.2000.094 Occurrence Handle1:CAS:528:DC%2BD3cXntlGmtr8%3D
ZJ Zhao YF Xu ZG Qian WH Tian XH Qian JJ Zhong (2004) Bioorg Med Chem Lett 14 4755 Occurrence Handle10.1016/j.bmcl.2004.06.082 Occurrence Handle1:CAS:528:DC%2BD2cXmvV2htLo%3D
ZG Qian ZJ Zhao YF Xu XH Qian JJ Zhong (2004) Biotech Bioeng 86 809 Occurrence Handle10.1002/bit.20092 Occurrence Handle1:CAS:528:DC%2BD2cXkslCgtL4%3D
ZG Qian ZJ Zhao WH Tian YF Xu JJ Zhong XH Qian (2004) Biotech Bioeng 85 595 Occurrence Handle10.1002/bit.20068
LC Desiree (1999) Crop Protection 18 267 Occurrence Handle10.1016/S0261-2194(99)00026-5
Binningen RS, Oberwil WK, Nyfeler R (1990) US Patent 4931581
JS Thaler AL Fidantsef (1999) J Chemical Ecology 25 7
YF Xu ZJ Zhao XH Qian ZG Qian WH Tian JJ Zhong (2006) J Agric Food Chem 54 8793 Occurrence Handle10.1021/jf0618574 Occurrence Handle1:CAS:528:DC%2BD28XhtVOqsLbL
P Schwenger P Bellosta I Vietor C Basilico EY Skolnik J Vilček (1997) Proc Natl Acad Sci 94 2869 Occurrence Handle10.1073/pnas.94.7.2869 Occurrence Handle1:CAS:528:DyaK2sXisVKgs7c%3D
E Flescher O Fingrut (2002) Leukemia 16 608 Occurrence Handle10.1038/sj.leu.2402419
R Rotem O Fingrut J Moskovitz E Flescher (2003) Leukemia 17 2230 Occurrence Handle10.1038/sj.leu.2403107 Occurrence Handle1:CAS:528:DC%2BD3sXotlartbo%3D
A Heyfets E Flescher (2007) Cancer Lett 250 300 Occurrence Handle10.1016/j.canlet.2006.10.013 Occurrence Handle1:CAS:528:DC%2BD2sXjvFGqur4%3D
E Flescher (2005) Anticancer Drugs 16 911 Occurrence Handle10.1097/01.cad.0000176501.63680.80 Occurrence Handle1:CAS:528:DC%2BD2MXpvFOqt7w%3D
R Rotem A Heyfets O Fingrut D Blickstein M Shaklai E Flescher (2005) Cancer Res 65 1984 Occurrence Handle10.1158/0008-5472.CAN-04-3091 Occurrence Handle1:CAS:528:DC%2BD2MXitVOjt7s%3D
E Flescher (2007) Cancer Lett 245 1 Occurrence Handle10.1016/j.canlet.2006.03.001 Occurrence Handle1:CAS:528:DC%2BD2sXht1eitA%3D%3D
W Kunz R Schurter T Maetzke (1997) Pestic Sci 50 275 Occurrence Handle10.1002/(SICI)1096-9063(199708)50:4<275::AID-PS593>3.0.CO;2-7 Occurrence Handle1:CAS:528:DyaK2sXltF2js70%3D
JH Kim SY Lee SY Oh SI Han HG Park MA Yoo HS Kang (2004) Oncol Rep 12 1233 Occurrence Handle1:CAS:528:DC%2BD2MXitFOlsQ%3D%3D
AJ Enyedi N Yalpanl P Silverman L Raskin (1992) Cell 70 879 Occurrence Handle10.1016/0092-8674(92)90239-9 Occurrence Handle1:CAS:528:DyaK38XmtVyrt7c%3D
H Kiyota M Saitoh T Oritani T Yoshihara (1996) Phytochemistry 42 1259 Occurrence Handle10.1016/0031-9422(96)00145-8 Occurrence Handle1:CAS:528:DyaK28Xkt1SksL4%3D
S Blechert C Bockelmann O Brümmer M Füβlein H Gundlach G Haider S Hölder TM Kutchan EW Weiler MH Zenk (1997) J Chem Soc Perkin Trans I 1 3549 Occurrence Handle10.1039/a702494k
T Krumm K Bandemer W Boland (1995) FEBS Lett 377 523 Occurrence Handle10.1016/0014-5793(95)01398-9 Occurrence Handle1:CAS:528:DyaK28Xnt12q
R Lauchli G Schüler W Boland (2002) Phytochemistry 61 807 Occurrence Handle10.1016/S0031-9422(02)00397-7 Occurrence Handle1:CAS:528:DC%2BD38XovFGguro%3D
G Schüler A Mithöfer IT Baldwin S Berger J Ebel JG Santos G Herrmann D Holscher R Kramell TM Kutchan H Maucher B Schneider I Stenzel C Wasternack W Boland (2004) FEBS Lett 563 17 Occurrence Handle10.1016/S0014-5793(04)00239-X
AC Cazalé MA Rouet-Mayer H Barbier-Brygoo Y Mathieu C Laurière (1998) Plant Physiol 116 659 Occurrence Handle10.1104/pp.116.2.659
Author information
Authors and Affiliations
Corresponding author
Additional information
Correspondence: Yufang Xu, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China.
Rights and permissions
About this article
Cite this article
Zhu, W., Zhao, Z. & Xu, Y. Derivatives of benzothiadiazole-7-carboxylates: synthesis and biological activity. Monatsh Chem 139, 1067–1071 (2008). https://doi.org/10.1007/s00706-008-0887-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-008-0887-3