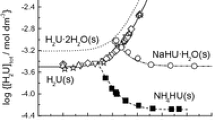
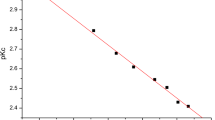
Summary.
(E)- and (Z)-Urocanic acids are endogenous chemicals in the normal mammalian skin. The first and the second thermodynamic dissociation constants (pK a1 and pK a2) of urocanic acid isomers were determined using UV spectrophotometry in aqueous solutions. The values with standard deviation (pK a1 = 3.43 ± 0.12 and pK a2 = 5.80 ± 0.04) and (pK a1 = 2.7 ± 0.3 and pK a2 = 6.65 ± 0.04) were obtained to (E)- and (Z)-urocanic acids, respectively. The second dissociations were studied also by potentiometric titration in aqueous sodium chloride solutions up to the isotonic salt concentration (0.154 mol dm−3), and the second thermodynamic dissociation constants as well as activity parameters for both isomers were determined at temperature 25°C and for (E)-urocanic acid also at 37°C. The obtained pK a2 values were close to those found by UV spectrophotometry. The equations for the calculation of the second stoichiometric dissociation constants of urocanic acid isomers on molality and molarity scale in aqueous sodium chloride solutions were derived. The obtained pK a1 and pK a2 values for (Z)-urocanic acid appear to be essentially lower than some previously reported values in literature.
Similar content being viewed by others
References
KC Moon K-P Wilhelm HI Maibach (1989) J Invest Dermatol 92 484
D Wilhelm P Elsner HI Maibach (1991) Acta Derm Venereol (Stockh) 71 123 Occurrence Handle1:STN:280:DyaK3M3mtlGlsg%3D%3D
S Seidenari G Giusti (1995) Acta Derm Venereol (Stockh) 75 429 Occurrence Handle1:STN:280:DyaK283jslSksw%3D%3D
F Rippke V Schreiner T Doering HI Maibach (2004) Am J Clin Dermatol 5 217 Occurrence Handle10.2165/00128071-200405040-00002
M Norval AA El-Ghorr (2002) Methods 28 63 Occurrence Handle10.1016/S1046-2023(02)00210-4 Occurrence Handle1:CAS:528:DC%2BD38XmslOhs78%3D
JD Roberts C Yu C Flanagan TR Birdseye (1982) J Am Chem Soc 104 3945 Occurrence Handle10.1021/ja00378a027 Occurrence Handle1:CAS:528:DyaL38XktlGhurk%3D
AH Mehler H Tabor (1953) J Biol Chem 201 775 Occurrence Handle1:CAS:528:DyaG3sXkvVWktw%3D%3D
JC Halle C Pichon F Terrier (1984) J Biol Chem 259 4142 Occurrence Handle1:CAS:528:DyaL2cXhslShtbo%3D
SC Zimmerman JS Korthals KD Cramer (1991) Tedrahedron 47 2649 Occurrence Handle10.1016/S0040-4020(01)81797-X Occurrence Handle1:CAS:528:DyaK3MXks1Ons7Y%3D
MJ Cloninger PA Frey (1998) Bioorg Chem 26 323 Occurrence Handle10.1006/bioo.1998.1115 Occurrence Handle1:CAS:528:DyaK1MXhtVKksb0%3D
A Albert EP Serjeant (1984) The Determination of Ionization Constants. A Laboratory Manual 3rd edn Chapman & Hall London
P Juusola (1999) Acta Polytech Scand Ch 269 1 Occurrence Handle1:CAS:528:DyaK1MXotFWqtrw%3D
M Alei LO Morgan WE Wageman TW Whaley (1980) J Am Chem Soc 102 2881 Occurrence Handle10.1021/ja00529a002 Occurrence Handle1:CAS:528:DyaL3cXkvFCmt7w%3D
H Morrison C Bernasconi G Pandey (1984) Photochem Photobiol 40 549 Occurrence Handle1:CAS:528:DyaL2MXis1an
JK Laihia H Lemmetyinen P Pasanen CT Jansen (1996) J Photochem Photobiol (B) 33 211 Occurrence Handle10.1016/1011-1344(95)07247-0 Occurrence Handle1:CAS:528:DyaK28XjsVymu7k%3D
JI Partanen PM Juusola V Verraes (2005) Can J Chem 83 46 Occurrence Handle10.1139/v04-164 Occurrence Handle1:CAS:528:DC%2BD2MXjtlShtLw%3D
DG Archer P Wang (1990) J Phys Chem Ref Data 19 371 Occurrence Handle1:CAS:528:DyaK3cXktlejtLc%3D Occurrence Handle10.1063/1.555853
J Partanen P Juusola P Minkkinen (1995) Acta Polytech Scand Ch 231 1
JI Partanen (1998) Ber Bunsenges Phys Chem 102 855 Occurrence Handle1:CAS:528:DyaK1cXjvVWlt7c%3D
JG Kirkwood (1934) J Chem Phys 2 351 Occurrence Handle10.1063/1.1749489 Occurrence Handle1:CAS:528:DyaA2cXksVWjsg%3D%3D
JG Kirkwood (1939) Chem Rev 24 233 Occurrence Handle10.1021/cr60078a004 Occurrence Handle1:CAS:528:DyaA1MXktVOntg%3D%3D
HS Harned WJ Hamer (1933) J Am Chem Soc 55 1933
JI Partanen PM Juusola PO Minkkinen V Verraes (2003) Can J Chem 81 1462 Occurrence Handle10.1139/v03-154 Occurrence Handle1:CAS:528:DC%2BD2cXhsFSkug%3D%3D
HS Harned GE Mannweiler (1935) J Am Chem Soc 57 1873 Occurrence Handle10.1021/ja01313a034 Occurrence Handle1:CAS:528:DyaA28XislCk
E Kreyszig (1979) Advanced Engineering Mathematics John Wiley & Sons New York 765
PM May DR Williams PW Linder RG Torrington (1982) Talanta 29 249 Occurrence Handle10.1016/0039-9140(82)80108-2 Occurrence Handle1:CAS:528:DyaL38XksVGht74%3D
JI Partanen PM Juusola PO Minkkinen (1999) Acta Chem Scand 53 547 Occurrence Handle1:CAS:528:DyaK1MXltlehtro%3D
JI Partanen PM Juusola PO Minkkinen (1999) J Solution Chem 28 413 Occurrence Handle10.1023/A:1022659912739 Occurrence Handle1:CAS:528:DyaK1MXkslyjtb0%3D
JI Partanen PM Juusola (2000) Fluid Phase Equilib 169 149 Occurrence Handle10.1016/S0378-3812(00)00311-3 Occurrence Handle1:CAS:528:DC%2BD3cXjtVWru7s%3D
JI Partanen PM Juusola (2000) Fluid Phase Equilib 173 135 Occurrence Handle10.1016/S0378-3812(00)00391-5 Occurrence Handle1:CAS:528:DC%2BD3cXmsFKrsLg%3D
King EJ (1945) J Am Chem Soc 67: 2178; King EJ (1951) J Am Chem Soc 73: 155
HS Harned BB Owen (1958) The Physical Chemistry of Electrolytic Solutions EditionNumber3 Reinhold Publishing Corporation New York 725
PM Krien M Kermici (2000) J Invest Dermatol 155 414 Occurrence Handle10.1046/j.1523-1747.2000.00083.x
E Schwarz (1977) Z Hautkr 52 IssueIDSuppl 2 59
A Taïeb D Montaudon P Loos P Donatien V Legrain A Cassaigne J Maleville (1991) NoChapterTitle JMP Czernielewski (Eds) Immunological and Pharmacological Aspects of Atopic and Contact Eczema Pharmacol Skin Basel 184
F Stäb U Hoppe G Sauermann (1994) J Invest Dermatol 102 666
L Juhlin B Shroot B Martin JC Caron (1986) Acta Derm Venereol 66 295 Occurrence Handle1:STN:280:DyaL2s%2FksVOqsg%3D%3D
B Schwarzinger H Falk (2004) Monatsh Chem 135 1297 Occurrence Handle10.1007/s00706-004-0219-1 Occurrence Handle1:CAS:528:DC%2BD2cXosFSjuro%3D
JI Partanen PM Juusola PO Minkkinen (1995) Acta Chem Scand 49 163 Occurrence Handle1:CAS:528:DyaK2MXltVGgsbc%3D Occurrence Handle10.3891/acta.chem.scand.49-0163
RP Buck S Rondinini AK Covington FGK Baucke CMA Brett MF Camoes MJT Milton T Mussini R Naumann KW Pratt P Spitzer GS Wilson (2002) Pure Appl Chem 74 2169 Occurrence Handle1:CAS:528:DC%2BD3sXhsFKnsLY%3D
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00706-007-0841-9.
Rights and permissions
About this article
Cite this article
Juusola, P., Minkkinen, P., Leino, L. et al. Determination of the Dissociation Constants of Urocanic Acid Isomers in Aqueous Solutions. Monatsh. Chem. 138, 951–965 (2007). https://doi.org/10.1007/s00706-007-0687-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-007-0687-1