Summary.
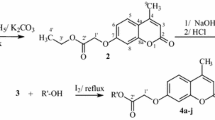
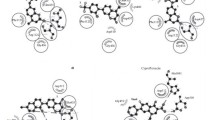
The one pot reaction preparation, spectral analysis, and molecular modeling experiments on the new 3,4-annelated coumarin systems with bioactivity associated structural features are described. These provided the insight into the equilibrium of the respective tautomeric forms making possible the reconciliation of previously published spectral data with the structure assignments as well as the correction of erroneously established structures. The synthesized compounds were tested in a standard disk diffusion assay and displayed strong to moderate antimicrobial activity, with a promising new lead prototype compound (7-amino-9-hydroxy-5-oxa-7a,8,11-triazacyclopenta[b]phenanthren-6-one (5)) possessing greater activity that the antibiotics Ampicillin and Nystatin. Antioxidant capacities of the compounds, determined spectrophotometrically using a phosphomolybdenum method, were greater, and in the case of compound 5 four times the activity of α-tocopherol acetate.
Similar content being viewed by others
References
TO Soine (1964) J Pharm Sci 53 231 Occurrence Handle1:CAS:528:DyaF2cXktVKrtbc%3D Occurrence Handle10.1002/jps.2600530302
R O’Kennedy RD Thornes (Eds) (1997) Coumarins – Biology, Applications and Mode of Action John Wiley & Sons Ltd. Chichester
Ojala T (2001) Biological Screening of Plant Coumarins, p 23–25, PhD thesis, Univeristy of Helsinki, Helsinki
LDS Yadav S Sharma A Vaish (1994) J Agric Food Chem 42 1352 Occurrence Handle1:CAS:528:DyaK2cXkt12is7k%3D Occurrence Handle10.1021/jf00042a020
DA Pratt GA DiLabio G Brigati GF Pedulli L Valgimigli (2001) J Am Chem Soc 123 4625 Occurrence Handle1:CAS:528:DC%2BD3MXislSqtbo%3D Occurrence Handle10.1021/ja005679l
L Kaljaj V Kaljaj S Dekic Lj Sokolic R Palic (1996) J Serb Chem Soc 61 419 Occurrence Handle1:CAS:528:DyaK28Xjs1Cnu7o%3D
S Chechi L Pecori Vettori M Mambagiotti (1968) Gaz Chim Ital 98 1448
S Govori V Kaljaj V Rapic L Kaljaj S Dakovic (2002) Heter Comm 8 129 Occurrence Handle1:CAS:528:DC%2BD38XltVGqu7g%3D
L Stefanovic-Kaljaj V Kaljaj M Trkovnik S Mladenovic (1989) J Serb Chem Soc 54 169 Occurrence Handle1:CAS:528:DyaK3cXhtFykt7o%3D
A Smith-Palmer J Stewart L Fyfe (1998) Lett Appl Microbiol 26 118 Occurrence Handle1:CAS:528:DyaK1cXisVKnsrc%3D Occurrence Handle10.1046/j.1472-765X.1998.00303.x
V Kaljaj M Trkovnik L Stefanovic-Kaljaj (1987) J Serb Chem Soc 52 183 Occurrence Handle1:CAS:528:DyaL1cXhsFSns74%3D
NCCLS (National Committee for Clinical Laboratory Standards) (1997) Performance standards for antimicrobial disk susceptibility test. Sixth ed. Approved Standard M2-A6, Wayne, PA
P Prieto M Pineda M Aguilar (1999) Anal Biochem 269 337 Occurrence Handle1:CAS:528:DyaK1MXis1Kmtbk%3D Occurrence Handle10.1006/abio.1999.4019
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radulović, N., Stojanović, G., Vukićević, R. et al. New 3,4-Annelated Coumarin Derivatives: Synthesis, Antimicrobial Activity, Antioxidant Capacity, and Molecular Modeling. Monatsh. Chem. 137, 1477–1486 (2006). https://doi.org/10.1007/s00706-006-0537-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-006-0537-6