Summary.
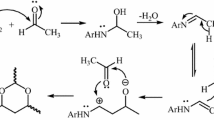
5-Isopropylidene-2,2-dimethyl-1,3-dioxane-4,6-dione (the condensation product of Meldrum’s acid and acetone) reacts smoothly with tert-butyl isocyanide in the presence of primary or secondary amines to produce N-tert-butyl-2,2-dimethylbutyramide derivatives and/or 1-tert-butyl-4,4-dimethyl-2,5-dioxopyrrolidine-3-carboxamides in good yields.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yavari, I., Habibi, A., Hosseini-Tabatabaei, M. et al. Reaction Between 5-Isopropylidene-2,2-dimethyl-1,3-dioxane-4,6-dione and tert-Butyl Isocyanide in the Presence of Primary or Secondary Amines. Monatshefte für Chemie 134, 1651–1658 (2003). https://doi.org/10.1007/s00706-003-0619-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-003-0619-7