Summary.
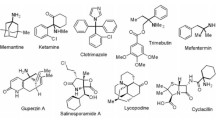
Various chiral enaminones and hydrazones, derived from chiral pool starting materials, such as L-aspartic acid, L-3-phenylalanine, (+)-camphor, and D-aldoses were employed as key-intermediates in the synthesis of functionalised heterocycles, such as aminomethylidene substituted tetramic acids, heteroaryl substituted phenethylamines and terpenes, and C-nucleosides.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svete, J. Ex-Chiral Pool Enaminones in the Synthesis of Functionalised Heterocycles. Monatshefte für Chemie 135, 629–647 (2004). https://doi.org/10.1007/s00706-003-0133-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-003-0133-y