Summary
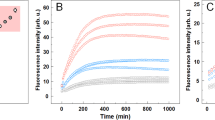
The human prion peptide PrP106–126 polymerizes in the presence of DNA both in its circular and linearized forms under solution conditions where the peptide alone does not polymerize. The polymerization process has been monitored by the increase in the fluorescence of anilino naphthalene sulfonic dye which detects the availability of the hydrophobic surface(s) in the aggregate as a consequence of polymerization. The polymerization is a nucleation dependent phenomenon as is evidenced from an existence of a lag period before the onset of the polymerization and a strong dependence of the polymerization on the prion peptide concentrations. The reaction is dependent on the pH as seen from rapid polymerization at pH 5 compared to the reaction at neutral pH where no polymerization is observed after a relatively long period of incubation. The polymer has been characterized as amyloid by using new absorbing and emitting species resulting from the interaction of the polymer with the amyloid specific fluorescent dye, Thioflavine S. This is probably the first demonstration that an endogenous macromolecule can influence the polymerization of a prion peptide. We have previously shown that there is a conformational change in the nucleic acid as a consequence of this interaction. This prion peptide is considered as a model to understand prion diseases as is evidenced from its toxicity towards primary brain cells in culture. The peptide encompasses one of the important amyloidogenic regions of the normal cellular prion protein. Demonstration of nucleic acid induced polymerization of the normal and scrapie prion isoforms accompanying a change in the nucleic acid conformation can establish a possible role of nucleic acid in prion disease.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received January 8, 1997 Accepted March 4, 1998
Rights and permissions
About this article
Cite this article
Nandi, P.K. Polymerization of human prion peptide HuPrP 106–126 to amyloid in nucleic acid solution. Arch. Virol. 143, 1251–1263 (1998). https://doi.org/10.1007/s007050050373
Published:
Issue Date:
DOI: https://doi.org/10.1007/s007050050373