Summary
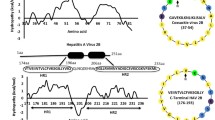
This work follows up on observations previously published concerning phosphatidylserine (PS) binding properties of synthetic peptides (p2) from the hydrophobic heptad repeats of the glycoprotein of viral haemorrhagic septicemia (VHS) rhabdovirus and the presence of similar repeats in the sequences of the glycoproteins of four separate rhabdoviruses. Similar p2-like peptides are now synthesized according to the corresponding cDNA sequences of infectious haematopoietic necrosis (IHN), rabies and vesicular stomatitis (VSV) viruses and shown to bind phosphatidylserine (PS) by solid-phase as well as from liquid-phase assays. The PS-binding peptides located in the amino-terminal part of the glycoproteins contained 3–5 contiguous heptad repeats (abcdefg) of hydrophobic amino acids (aa) in positions a and d followed by a short aa stretch containing positively charged aa and not belonging to the heptad repeats. The rhabdoviral PS-binding regions had low sequence variability among the members of each of the rhabdoviral genus but show no sequence similarity among the different genera.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received February 20, 1997 Accepted May 26, 1997
Rights and permissions
About this article
Cite this article
Coll, J.M. Synthetic peptides from the heptad repeats of the glycoproteins of rabies, vesicular stomatitis and fish rhabdoviruses bind phosphatidylserine. Arch. Virol. 142, 2089–2097 (1997). https://doi.org/10.1007/s007050050227
Published:
Issue Date:
DOI: https://doi.org/10.1007/s007050050227