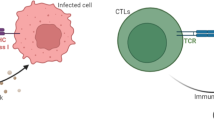
Abstract
The "Shock and Kill" method is being considered as a potential treatment for eradicating HIV-1 and achieving a functional cure for acquired immunodeficiency syndrome (AIDS). This approach involves using latency-reversing agents (LRAs) to activate human immunodeficiency virus (HIV-1) transcription in latent cells, followed by treatment with antiviral drugs to kill these cells. Although LRAs have shown promise in HIV-1 patient research, their widespread clinical use is hindered by side effects and limitations. In this review, we categorize and explain the mechanisms of these agonists in activating HIV-1 in vivo and discuss their advantages and disadvantages. In the future, combining different HIV-1 LRAs may overcome their respective shortcomings and facilitate a functional cure for HIV-1.




Similar content being viewed by others
Change history
16 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00705-024-05972-1
References
Menéndez-Arias L, Delgado R (2022) Update and latest advances in antiretroviral therapy. Trends Pharmacol Sci 43(1):16–29
Chen J, Zhou T, Zhang Y, Luo S, Chen H, Chen D, Li C, Li W (2022) The reservoir of latent HIV. Front Cell Infect Microbiol 12:945956
Kumar A, Abbas W, Herbein G (2014) HIV-1 latency in monocytes/macrophages. Viruses 6(4):1837–1860
Kruize Z, Kootstra NA (2019) The Role of Macrophages in HIV-1 Persistence and Pathogenesis. Front Microbiol 10:2828
Balan S, Saxena M, Bhardwaj N (2019) Dendritic cell subsets and locations. Int Rev Cell Mol Biol 348:1–68
Yin X, Chen S, Eisenbarth SC (2021) Dendritic Cell Regulation of T Helper Cells. Annu Rev Immunol 39:759–790
Yu HJ, Reuter MA, McDonald D (2008) HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog 4 (8), e1000134
Carter CC, McNamara LA, Onafuwa-Nuga A, Shackleton M, Riddell Jt, Bixby D, Savona MR, Morrison SJ, Collins KL (2011) HIV-1 utilizes the CXCR4 chemokine receptor to infect multipotent hematopoietic stem and progenitor cells. Cell Host Microbe 9(3):223–234
Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell Jt, Bixby D, Savona MR, Collins KL (2010) HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med 16(4):446–451
Bandera A, Gori A, Clerici M, Sironi M (2019) Phylogenies in ART: HIV reservoirs, HIV latency and drug resistance. Curr Opin Pharmacol 48:24–32
Baba M (2004) [Recent progress in anti-HIM-1 research]. Uirusu 54(1):59–66
Deeks SG (2012) Shock and kill. Nature 487(7408):439–440
Boateng AT, Abaidoo-Myles A, Bonney EY, Kyei GB (2022) Isoform-Selective Versus Nonselective Histone Deacetylase Inhibitors in HIV Latency Reversal. AIDS Res Hum Retroviruses 38(8):615–621
Varier RA, Kundu TK (2006) Chromatin modifications (acetylation/ deacetylation/ methylation) as new targets for HIV therapy. Curr Pharm Design 12(16):1975–1993
Archin NM, Kirchherr JL, Sung JA, Clutton G, Sholtis K, Xu Y, Allard B, Stuelke E, Kashuba AD, Kuruc JD, Eron J, Gay CL, Goonetilleke N, Margolis DM (2017) Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Investig 127(8):3126–3135
Glozak MA, Seto E (2007) Histone deacetylases and cancer. Oncogene 26(37):5420–5432
Vandergeeten C, Quivy V, Moutschen M, Van Lint C, Piette J, Legrand-Poels S (2007) HIV-1 protease inhibitors do not interfere with provirus transcription and host cell apoptosis induced by combined treatment TNF-alpha + TSA. Biochem Pharmacol 73(11):1738–1748
Lin S, Zhang Y, Ying H, Zhu H (2011) HIV-1 reactivation induced by apicidin involves histone modification in latently infected cells. Curr HIV Res 9(4):202–208
Newhard W, Patel M, Cassaday J, Ballard J, Squadroni B, Wu G, Liu J, Yu W, Kozlowski J, Zuck P, Howell B, Hazuda D, Vargo R, Barnard R (2021) In Vitro Pharmacokinetic/Pharmacodynamic Modeling of HIV Latency Reversal by Novel HDAC Inhibitors Using an Automated Platform. SLAS discovery: advancing life sciences R & D 26(5):642–654
Garrido C, Tolstrup M, Søgaard OS, Rasmussen TA, Allard B, Soriano-Sarabia N, Archin NM, Margolis DM (2019) In-vivo administration of histone deacetylase inhibitors does not impair natural killer cell function in HIV + individuals. AIDS 33(4):605–613
Kiefer HL, Hanley TM, Marcello JE, Karthik AG, Viglianti GA (2004) Retinoic acid inhibition of chromatin remodeling at the human immunodeficiency virus type 1 promoter. Uncoupling of histone acetylation and chromatin remodeling. J Biol Chem 279(42):43604–43613
Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Breslow R, Pavletich NP (1999) Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401(6749):188–193
He G, Margolis DM (2002) Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Mol Cell Biol 22(9):2965–2973
Iveland TS, Hagen L, Sharma A, Sousa MML, Sarno A, Wollen KL, Liabakk NB, Slupphaug G (2020) HDACi mediate UNG2 depletion, dysregulated genomic uracil and altered expression of oncoproteins and tumor suppressors in B- and T-cell lines. J translational Med 18(1):159
Bose P, Dai Y, Grant S (2014) Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights. Pharmacol Ther 143(3):323–336
Jeng MY, Ali I, Ott M (2015) Manipulation of the host protein acetylation network by human immunodeficiency virus type 1. Crit Rev Biochem Mol Biol 50(4):314–325
Beliakova-Bethell N, Mukim A, White CH, Deshmukh S, Abewe H, Richman DD, Spina CA (2019) Histone deacetylase inhibitors induce complex host responses that contribute to differential potencies of these compounds in HIV reactivation. J Biol Chem 294(14):5576–5589
McMahon DK, Zheng L, Cyktor JC, Aga E, Macatangay BJ, Godfrey C, Para M, Mitsuyasu RT, Hesselgesser J, Dragavon J, Dobrowolski C, Karn J, Acosta EP, Gandhi RT, Mellors JW (2021) Phase 1/2 Randomized, Placebo-Controlled Trial of Romidespin in Persons With HIV-1 on Suppressive Antiretroviral Therapy. J Infect Dis 224(4):648–656
Zhang H, Li X, Zhang Q, Yang F, Chu X, Zhang D, Wang L, Gong Z (2017) Role of histone deacetylase expression levels and activity in the inflammatory responses of patients with chronic hepatitis B. Mol Med Rep 15(5):2744–2752
Shah MH, Binkley P, Chan K, Xiao J, Arbogast D, Collamore M, Farra Y, Young D, Grever M (2006) Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res 12(13):3997–4003
Molife R, Fong P, Scurr M, Judson I, Kaye S, de Bono J (2007) HDAC inhibitors and cardiac safety. Clin Cancer Res 13 (3), 1068; author reply 1068-9
Bai M, Cui M, Li M, Yao X, Wu Y, Zheng L, Sun L, Song Q, Wang S, Liu L, Yu C, Huang Y (2021) Discovery of a novel HDACi structure that inhibits the proliferation of ovarian cancer cells in vivo and in vitro. Int J Biol Sci 17(13):3493–3507
Kroesen M, Gielen P, Brok IC, Armandari I, Hoogerbrugge PM, Adema GJ (2014) HDAC inhibitors and immunotherapy; a double edged sword? Oncotarget 5(16):6558–6572
Subramanian S, Bates SE, Wright JJ, Espinoza-Delgado I, Piekarz RL (2010) Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals (Basel Switzerland) 3(9):2751–2767
du Chéné I, Basyuk E, Lin YL, Triboulet R, Knezevich A, Chable-Bessia C, Mettling C, Baillat V, Reynes J, Corbeau P, Bertrand E, Marcello A, Emiliani S, Kiernan R, Benkirane M (2007) Suv39H1 and HP1gamma are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. Embo j 26(2):424–435
Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, Aunis D, Rohr O (2007) Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. Embo j 26(2):412–423
Bernhard W, Barreto K, Saunders A, Dahabieh MS, Johnson P, Sadowski I (2011) The Suv39H1 methyltransferase inhibitor chaetocin causes induction of integrated HIV-1 without producing a T cell response. FEBS Lett 585(22):3549–3554
Bouchat S, Gatot JS, Kabeya K, Cardona C, Colin L, Herbein G, De Wit S, Clumeck N, Lambotte O, Rouzioux C, Rohr O, Van Lint C (2012) Histone methyltransferase inhibitors induce HIV-1 recovery in resting CD4(+) T cells from HIV-1-infected HAART-treated patients. AIDS 26(12):1473–1482
Rombo R, Weiher H, Schmidt-Wolf IG (2016) Effect of chaetocin on renal cell carcinoma cells and cytokine-induced killer cells. German medical science: GMS e-journal 14, Doc04
Imai K, Togami H, Okamoto T (2010) Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294. J Biol Chem 285(22):16538–16545
Chase KA, Feiner B, Ramaker MJ, Hu E, Rosen C, Sharma RP (2019) Examining the effects of the histone methyltransferase inhibitor BIX-01294 on histone modifications and gene expression in both a clinical population and mouse models. PLoS ONE 14 (6), e0216463
Zhang X, Justice AC, Hu Y, Wang Z, Zhao H, Wang G, Johnson EO, Emu B, Sutton RE, Krystal JH, Xu K (2016) Epigenome-wide differential DNA methylation between HIV-infected and uninfected individuals. Epigenetics 11(10):750–760
van der Wijst MG, Venkiteswaran M, Chen H, Xu GL, Plösch T, Rots MG (2015) Local chromatin microenvironment determines DNMT activity: from DNA methyltransferase to DNA demethylase or DNA dehydroxymethylase. Epigenetics 10(8):671–676
Pierard V, Guiguen A, Colin L, Wijmeersch G, Vanhulle C, Van Driessche B, Dekoninck A, Blazkova J, Cardona C, Merimi M, Vierendeel V, Calomme C, Nguyên TL, Nuttinck M, Twizere JC, Kettmann R, Portetelle D, Burny A, Hirsch I, Rohr O, Van Lint C (2010) DNA cytosine methylation in the bovine leukemia virus promoter is associated with latency in a lymphoma-derived B-cell line: potential involvement of direct inhibition of cAMP-responsive element (CRE)-binding protein/CRE modulator/activation transcription factor binding. J Biol Chem 285(25):19434–19449
Bouchat S, Delacourt N, Kula A, Darcis G, Van Driessche B, Corazza F, Gatot JS, Melard A, Vanhulle C, Kabeya K, Pardons M, Avettand-Fenoel V, Clumeck N, De Wit S, Rohr O, Rouzioux C, Van Lint C (2016) Sequential treatment with 5-aza-2'-deoxycytidine and deacetylase inhibitors reactivates HIV-1. EMBO Mol Med 8(2):117–138
Abdel-Hameed EA, Ji H, Shata MT (2016) HIV-Induced Epigenetic Alterations in Host Cells. Adv Exp Med Biol 879:27–38
Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, Alousi AM, Hosing C, Giralt S, Rondon G, Woodworth G, Champlin RE (2020) A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv 4(21):5580–5588
Fernandez G, Zeichner SL (2010) Cell line-dependent variability in HIV activation employing DNMT inhibitors. Virol J 7:266
Stewart DJ, Donehower RC, Eisenhauer EA, Wainman N, Shah AK, Bonfils C, MacLeod AR, Besterman JM, Reid GK (2003) A phase I pharmacokinetic and pharmacodynamic study of the DNA methyltransferase 1 inhibitor MG98 administered twice weekly. Annals of oncology: official journal of the European Society for Medical Oncology 14(5):766–774
Winquist E, Knox J, Ayoub JP, Wood L, Wainman N, Reid GK, Pearce L, Shah A, Eisenhauer E (2006) Phase II trial of DNA methyltransferase 1 inhibition with the antisense oligonucleotide MG98 in patients with metastatic renal carcinoma: a National Cancer Institute of Canada Clinical Trials Group investigational new drug study. Invest New Drugs 24(2):159–167
Plummer R, Vidal L, Griffin M, Lesley M, de Bono J, Coulthard S, Sludden J, Siu LL, Chen EX, Oza AM, Reid GK, McLeod AR, Besterman JM, Lee C, Judson I, Calvert H, Boddy AV (2009) Phase I study of MG98, an oligonucleotide antisense inhibitor of human DNA methyltransferase 1, given as a 7-day infusion in patients with advanced solid tumors. Clin Cancer Res 15(9):3177–3183
Zaborowska J, Isa NF, Murphy S (2016) P-TEFb goes viral. BioEssays 38(Suppl 1):S75–85
Franco LC, Morales F, Boffo S, Giordano A (2018) CDK9: A key player in cancer and other diseases. J Cell Biochem 119(2):1273–1284
Chen D, Wang H, Aweya JJ, Chen Y, Chen M, Wu X, Chen X, Lu J, Chen R, Liu M (2016) HMBA Enhances Prostratin-Induced Activation of Latent HIV-1 via Suppressing the Expression of Negative Feedback Regulator A20/TNFAIP3 in NF-κB Signaling. Biomed Res Int 2016. 5173205
Contreras X, Barboric M, Lenasi T, Peterlin BM (2007) HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog 3(10):1459–1469
Cheng Y, Jin Z, Agarwal R, Ma K, Yang J, Ibrahim S, Olaru AV, David S, Ashktorab H, Smoot DT, Duncan MD, Hutcheon DF, Abraham JM, Meltzer SJ, Mori Y (2012) LARP7 is a potential tumor suppressor gene in gastric cancer. Lab Invest 92(7):1013–1019
Dey A, Chao SH, Lane DP (2007) HEXIM1 and the control of transcription elongation: from cancer and inflammation to AIDS and cardiac hypertrophy. Cell Cycle 6(15):1856–1863
Claudio PP, Cui J, Ghafouri M, Mariano C, White MK, Safak M, Sheffield JB, Giordano A, Khalili K, Amini S, Sawaya BE (2006) Cdk9 phosphorylates p53 on serine 392 independently of CKII. J Cell Physiol 208(3):602–612
Simone C, Bagella L, Bellan C, Giordano A (2002) Physical interaction between pRb and cdk9/cyclinT2 complex. Oncogene 21(26):4158–4165
Fujinaga K (2020) P-TEFb as A Promising Therapeutic Target. Molecules 25 (4)
Gohda J, Suzuki K, Liu K, Xie X, Takeuchi H, Inoue JI, Kawaguchi Y, Ishida T (2018) BI-2536 and BI-6727, dual Polo-like kinase/bromodomain inhibitors, effectively reactivate latent HIV-1. Sci Rep 8(1):3521
Darcis G, Kula A, Bouchat S, Fujinaga K, Corazza F, Ait-Ammar A, Delacourt N, Melard A, Kabeya K, Vanhulle C, Van Driessche B, Gatot JS, Cherrier T, Pianowski LF, Gama L, Schwartz C, Vila J, Burny A, Clumeck N, Moutschen M, De Wit S, Peterlin BM, Rouzioux C, Rohr O, Van Lint C (2015) An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1 + JQ1 and Ingenol-B + JQ1 to Potently Reactivate Viral Gene Expression. PLoS Pathog 11(7):e1005063
Sánchez-Ventura J, Amo-Aparicio J, Navarro X, Penas C (2019) BET protein inhibition regulates cytokine production and promotes neuroprotection after spinal cord injury. J Neuroinflamm 16(1):124
Domínguez-Andrés J, Ferreira AV, Jansen T, Smithers N, Prinjha RK, Furze RC, Netea MG (2019) Bromodomain inhibitor I-BET151 suppresses immune responses during fungal-immune interaction. Eur J Immunol 49(11):2044–2050
Salahong T, Schwartz C, Sungthong R, Are BET (2021) Inhibitors yet Promising Latency-Reversing Agents for HIV-1 Reactivation in AIDS Therapy? Viruses 13 (6)
Lu P, Shen Y, Yang H, Wang Y, Jiang Z, Yang X, Zhong Y, Pan H, Xu J, Lu H, Zhu H (2017) BET inhibitors RVX-208 and PFI-1 reactivate HIV-1 from latency. Sci Rep 7(1):16646
Li G, Zhang Z, Reszka-Blanco N, Li F, Chi L, Ma J, Jeffrey J, Cheng L, Su L (2019) Specific Activation in Vivo of HIV-1 by a Bromodomain Inhibitor from Monocytic Cells in Humanized Mice under Antiretroviral Therapy. J Virol 93:12
Doyon G, Zerbato J, Mellors JW, Sluis-Cremer N (2013) Disulfiram reactivates latent HIV-1 expression through depletion of the phosphatase and tensin homolog. AIDS 27(2):F7–f11
Xing S, Bullen CK, Shroff NS, Shan L, Yang HC, Manucci JL, Bhat S, Zhang H, Margolick JB, Quinn TC, Margolis DM, Siliciano JD, Siliciano RF (2011) Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4 + T cell model without inducing global T cell activation. J Virol 85(12):6060–6064
Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN, Emad F, Buckheit R 3rd;, McCance-Katz EF, Lai J, Kennedy M, Chander G, Siliciano RF, Siliciano JD, Deeks SG (2014) A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect diseases: official publication Infect Dis Soc Am 58(6):883–890
Lee SA, Elliott JH, McMahon J, Hartogenesis W, Bumpus NN, Lifson JD, Gorelick RJ, Bacchetti P, Deeks SG, Lewin SR, Savic RM (2019) Population Pharmacokinetics and Pharmacodynamics of Disulfiram on Inducing Latent HIV-1 Transcription in a Phase IIb Trial. Clin Pharmacol Ther 105(3):692–702
Deng L, Zeng Q, Wang M, Cheng A, Jia R, Chen S, Zhu D, Liu M, Yang Q, Wu Y, Zhao X, Zhang S, Liu Y, Yu Y, Zhang L, Chen X (2018) Suppression of NF-κB Activity: A Viral Immune Evasion Mechanism. Viruses 10:8
Khan SZ, Gasperino S, Zeichner SL (2019) Nuclear Transit and HIV LTR Binding of NF-κB Subunits Held by IκB Proteins: Implications for HIV-1 Activation. Viruses 11 (12)
Fernandez G, Zaikos TD, Khan SZ, Jacobi AM, Behlke MA, Zeichner SL (2013) Targeting IκB proteins for HIV latency activation: the role of individual IκB and NF-κB proteins. J Virol 87(7):3966–3978
Mbonye U, Leskov K, Shukla M, Valadkhan S, Karn J (2021) Biogenesis of P-TEFb in CD4 + T cells to reverse HIV latency is mediated by protein kinase C (PKC)-independent signaling pathways. PLoS Pathog 17 (9), e1009581
Okoye AA, Fromentin R, Takata H, Brehm JH, Fukazawa Y, Randall B, Pardons M, Tai V, Tang J, Smedley J, Axthelm M, Lifson JD, Picker LJ, Favre D, Trautmann L, Chomont N (2022) The ingenol-based protein kinase C agonist GSK445A is a potent inducer of HIV and SIV RNA transcription. PLoS Pathog 18 (1), e1010245
Gutiérrez C, Serrano-Villar S, Madrid-Elena N, Pérez-Elías MJ, Martín ME, Barbas C, Ruipérez J, Muñoz E, Muñoz-Fernández MA, Castor T, Moreno S (2016) Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 30(9):1385–1392
Gama L, Abreu CM, Shirk EN, Price SL, Li M, Laird GM, Pate KA, Wietgrefe SW, O'Connor SL, Pianowski L, Haase AT, Van Lint C, Siliciano RF, Clements JE (2017) Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS 31(1):5–14
Marsden MD, Loy BA, Wu X, Ramirez CM, Schrier AJ, Murray D, Shimizu A, Ryckbosch SM, Near KE, Chun TW, Wender PA, Zack JA (2017) In vivo activation of latent HIV with a synthetic bryostatin analog effects both latent cell kick and kill in strategy for virus eradication. PLoS Pathog 13 (9), e1006575
Beans EJ, Fournogerakis D, Gauntlett C, Heumann LV, Kramer R, Marsden MD, Murray D, Chun TW, Zack JA, Wender PA (2013) Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci USA 110(29):11698–11703
DeChristopher BA, Loy BA, Marsden MD, Schrier AJ, Zack JA, Wender PA (2012) Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat Chem 4(9):705–710
Clerici M, Galli M, Bosis S, Gervasoni C, Moroni M, Norbiato G (2000) Immunoendocrinologic abnormalities in human immunodeficiency virus infection. Ann N Y Acad Sci 917:956–961
Nakano H (2004) Signaling crosstalk between NF-kappaB and JNK. Trends Immunol 25(8):402–405
Khan KA, Abbas W, Varin A, Kumar A, Di Martino V, Dichamp I, Herbein G (2013) HIV-1 Nef interacts with HCV Core, recruits TRAF2, TRAF5 and TRAF6, and stimulates HIV-1 replication in macrophages. J Innate Immun 5(6):639–656
Herbein G (2016) HIV-1 Nef: An Intimate Interplay. EBioMedicine 13:25–26
Reuse S, Calao M, Kabeya K, Guiguen A, Gatot JS, Quivy V, Vanhulle C, Lamine A, Vaira D, Demonte D, Martinelli V, Veithen E, Cherrier T, Avettand V, Poutrel S, Piette J, de Launoit Y, Moutschen M, Burny A, Rouzioux C, De Wit S, Herbein G, Rohr O, Collette Y, Lambotte O, Clumeck N, Van Lint C (2009) Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PLoS ONE 4 (6), e6093
Cummins NW, Badley AD (2010) Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell Death Dis 1(11):e99
Fletcher CV, Dyavar SR, Acharya A, Byrareddy SN (2021) The Contributions of Clinical Pharmacology to HIV Cure Research. Clin Pharmacol Ther 110(2):334–345
Kim Y, Anderson JL, Lewin SR (2018) Getting the Kill into Shock and Kill: Strategies to Eliminate Latent HIV. Cell Host Microbe 23(1):14–26
Garcia-Vidal E, Badia R, Pujantell M, Castellví M, Felip E, Clotet B, Riveira-Muñoz E, Ballana E, Esté JA (2019) Dual effect of the broad spectrum kinase inhibitor midostaurin in acute and latent HIV-1 infection. Antiviral Res 168:18–27
Cary DC, Fujinaga K, Peterlin BM (2016) Molecular mechanisms of HIV latency. J Clin Investig 126(2):448–454
Yang W, Sun Z, Hua C, Wang Q, Xu W, Deng Q, Pan Y, Lu L, Jiang S (2018) Chidamide, a histone deacetylase inhibitor-based anticancer drug, effectively reactivates latent HIV-1 provirus. Microbes Infect 20(9–10):626–634
Kuai Q, Lu X, Qiao Z, Wang R, Wang Y, Ye S, He M, Wang Y, Zhang T, Wu H, Ren S, Yu Q (2018) Histone deacetylase inhibitor chidamide promotes reactivation of latent human immunodeficiency virus by introducing histone acetylation. J Med Virol 90(9):1478–1485
Li JH, Ma J, Kang W, Wang CF, Bai F, Zhao K, Yao N, Liu Q, Dang BL, Wang BW, Wei QQ, Kang WZ, Sun YT (2020) The histone deacetylase inhibitor chidamide induces intermittent viraemia in HIV-infected patients on suppressive antiretroviral therapy. HIV Med 21(11):747–757
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81802975), the Zhejiang Provincial Natural Science Foundation (Grant No. LY24H190004), and the Zhejiang Medical and Health Science and Technology Project (Grant No. 2019KY361, 2020KY101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Carolina Scagnolari
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Affiliations of authors updated. Funding infomation ammended.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Jiang, Y., Zhong, X. et al. Characteristics and mechanisms of latency-reversing agents in the activation of the human immunodeficiency virus 1 reservoir. Arch Virol 168, 301 (2023). https://doi.org/10.1007/s00705-023-05931-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-023-05931-2