Abstract
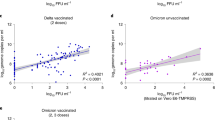
The emergence and evolution of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variants that could compromise vaccine efficacy (VE) with re-infections in immunized individuals have necessitated continuous surveillance of VE. Here, the occurrence and dynamics of SARS-CoV-2 infections in the context of vaccination during the second wave of infection in Mumbai were evaluated. RT-PCR cycle threshold (Ct) values of the open reading frame (ORF)/envelope (E)/nucleocapsid (N) genes obtained from a total of 42415 samples, comprising unvaccinated (96.88%) and vaccinated cases (3.12%) were analyzed between December 28, 2020, and August 30, 2021. A lower incidence of SARS-CoV-2 infection in fully vaccinated cases (5.07%) compared to partially vaccinated cases (6.5%) and unvaccinated cases (13.453%) was recorded. VE was significant after the first dose of vaccination (ORF gene p-value = 0.003429, and E/N gene p-value = 0.000866). Furthermore, VE was observed to be significant when the post-immunization (first dose) period was stratified to within 30 days (ORF gene p-value = 0.0094 and E/N gene p-value = 0.0023) and to 60 days following the second dose of vaccination (ORF gene p-value = 0.0238). Also, significantly higher efficacy was observed within individuals receiving two doses compared to a single dose (ORF gene p-value = 0.0132 and E/N gene p-value = 0.0387). The emergence of breakthrough infections was also evident (odds ratio= 0.34; 95% confidence interval= 0.27–0.43). Interestingly, viral loads trended towards being higher in some groups of partially vaccinated individuals compared to completely vaccinated and unvaccinated populations. Finally, our results delineated a significantly higher incidence of SARS-CoV-2 acquisition in males, asymptomatic individuals, individuals with comorbidities, and those who were unvaccinated.


Similar content being viewed by others
Availability of data and material:
Data made available online
References
Cucinotta D, Vanelli M (2020) WHO declares COVID-19 a pandemic. Acta Biomed 91:157–160. https://doi.org/10.23750/abm.v91i1.9397
Brodeur A, Gray D, Islam A, Bhuiyan S (2021) A literature review of the economics of COVID-19. J Econ Surv 35(4):1007–1044. https://doi.org/10.1111/joes.12423
WHO (2021a) SARS-CoV-2 Delta variant now dominant in much of European region; efforts must be reinforced to prevent transmission, warns WHO Regional Office for Europe and ECDC. http://www.euro.who.int/en/media-centre/sections/press-releases/2021/sars-cov-2-delta-variant-now-dominantin-much-of-european-region-efforts-must-be-reinforced-toprevent-transmission,-warns-who-regional-office-for-europeand-ecdc. Accessed on 8th March 2022
WHO, Technical Advisory Group on SARS-CoV-2 Virus Evolution (TAG-VE) (2021b). Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. http://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variantof-concern. Accessed on 8th March 2022
Dong Ensheng Du, Hongru G (2020) Lauren An interactive web-based dashboard to track COVID-19 in real-time. Lancet Infect Dis 20(5):533–34. https://ourworldindata.org/explorers/coronavirus-data-explorer. Accessed on May 16, 2022
Rajeshbhai SP, Dhar SS, Shalabh (2022) Fourth wave of COVID-19 in India: Statistical forecasting. medRxiv 2022.02.23.22271382. doi: https://doi.org/10.1101/2022.02.23.22271382
Munne K, Bhanothu V, Bhor V et al (2021) Detection of SARS-CoV-2 infection by RT-PCR test: factors influencing interpretation of results. Virusdisease 32(2):1–3. https://doi.org/10.1007/s13337-021-00692-5
Peiris M, Leung GM (2020) What can we expect from first-generation COVID-19 vaccines? Lancet 396(1026):1467–1469. https://doi.org/10.1016/S0140-6736(20)31976-0
WHO (2022) COVID-19 vaccines. Status of COVID-19 vaccines within WHO EUL/PQ evaluation process. https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_08November2022.pdf Accessed on 18th November 2022
COVID-19 vaccines approved in the country (2023) https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=OTA4MQ==. Accessed on January 20, 2023
Edouard M, Ritchie H, Rodés-Guirao L et al (2020) Coronavirus Pandemic (COVID-19). Published online at OurWorldInData.org. Retrieved from: ‘https://ourworldindata.org/coronavirus’ [Online Resource]. https://ourworldindata.org/covid-vaccinations#citation Accessed on December 19, 2022
Accorsi EK, Britton A, Fleming-Dutra KE et al (2022) Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA 327(7):639–651. https://doi.org/10.1001/jama.2022.0470
Pajon R, Paila YD, Girard B et al (2022) Initial analysis of viral dynamics and circulating viral variants during the mRNA-1273 Phase 3 COVE trial. Nat Med 28:823–830. https://doi.org/10.1038/s41591-022-01679-5
Singanayagam A, Hakki S, Dunning J et al (2022) ATACCC Study Investigators. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 22(2):183–195. doi: https://doi.org/10.1016/S1473-3099(21)00648-4. Erratum in: Lancet Infect Dis. 2021;21(12): e363
Zeng G, Wu Q, Pan H et al (2022) Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis 22(4):483–495. https://doi.org/10.1016/S1473-3099(21)00681-2
Kustin T, Harel N, Finkel U et al (2021) Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med 27:1379–1384. https://doi.org/10.1038/s41591-021-01413-7
Hacisuleyman E, Hale C, Saito Y et al (2021) Vaccine breakthrough infections with SARS-CoV‐2 variants. N Engl J Med 384(23):2212–2218. https://doi.org/10.1056/NEJMoa2105000
Chemaitelly H, Yassine HM, Benslimane FM et al (2021) mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 27:1614–1621. https://doi.org/10.1038/s41591-021-01446-y
Chen JM, Sun YX, Chen JW (2020) Potential for elimination of SAR-CoV-2 through vaccination as inspired by elimination of multiple influenza viruses through natural pandemics or mass vaccination. J Med Virol 92(11):2453–2457. https://doi.org/10.1002/jmv.26162
Bobdey S, Kaushik SK, Sahu R et al (2021) Effectiveness of ChAdOx1 nCOV-19 Vaccine: Experience of a tertiary care institute. Med J Armed Forces India 77(Suppl 2):S271–S277. https://doi.org/10.1016/j.mjafi.2021.06.006
Dhumal S, Patil A, More A et al (2022) SARS-COV-2 reinfection after previous infection and vaccine breakthrough infection through the second wave of pandemic in India: An observational study. Int J Infect Dis 118:95–103. https://doi.org/10.1016/j.ijid.2022.02.037
Abhilash KPP, Mathiyalagan P, Krishnaraj VRK et al (2022) Impact of prior vaccination with Covishield ™ and Covaxin ® on mortality among symptomatic COVID-19 patients during the second wave of the pandemic in South India during April and May 2021: a cohort study. Vaccine 40(13):2107–2113. https://doi.org/10.1016/j.vaccine.2022.02.023
Malhotra S, Mani K, Lodha R et al (2022) COVID Reinfection AIIMS Consortium. SARS-CoV-2 reinfection rate and estimated effectiveness of the inactivated whole virion vaccine BBV152 against reinfection among health care workers in New Delhi, India. JAMA Netw Open 5(1):e2142210. https://doi.org/10.1001/jamanetworkopen.2021.42210
Parida SP, Sahu DP, Singh AK et al (2022) Adverse events following immunization of COVID-19 (Covaxin) vaccine at a tertiary care center of India. J Med Virol 94(6):2453–2459. https://doi.org/10.1002/jmv.27655
Singanayagam A, Patel M, Charlett A et al (2020) Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 25(32):2001483. https://doi.org/10.2807/1560-7917.ES.2020.25.32.2001483. Erratum in: Euro Surveill. 2021;26(7): PMID: 32794447
Kuhlmann C, Mayer CK, Claassen M et al (2022) Breakthrough infections with SARS-CoV-2 Omicron despite mRNA vaccine booster dose. Correspondence Lancet 399(10325):625–626. https://doi.org/10.1016/S0140-6736(22)00090-3
WHO (2022) Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed on 20th April 2022
Kreier F (2022) Deltacron: the story of the variant that wasn’t. Nature 602(7895):19. https://doi.org/10.1038/d41586-022-00149-9
Kumar S, Karuppanan K, Subramaniam G (2022) Omicron (BA.1) and sub-variants (BA.1, BA.2 and BA.3) of SARS-CoV-2 spike infectivity and pathogenicity: A comparative sequence and structural-based computational assessment. https://doi.org/10.1101/2022.02.11.480029. bioRxiv 2022.02.11.480029
Klaassen F, Chitwood MH, Cohen T et al (2022) Population immunity to pre-Omicron and Omicron SARS-CoV-2 variants in US states and counties through December 1, 2021. medRxiv.2021.12.23.21268272. https://doi.org/10.1101/2021.12.23.21268272
Dhar MS, Marwal R, Vs R et al (2021) Indian SARS-CoV-2 Genomics Consortium (INSACOG); Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. Science 374(6570):995–999. https://doi.org/10.1126/science.abj9932
Delanghe JR, Speeckaert MM, De Buyzere ML (2020) The host’s angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin Chim Acta 505:192–193. https://doi.org/10.1016/j.cca.2020.03.031
Lipsitch M, Dean NE (2020) Understanding COVID-19 vaccine efficacy. Science 370(6518):763–765. https://doi.org/10.1126/science.abe5938
Davies NG, Abbott S, Barnard RC, COVID-19 Genomics UK (COG-UK) Consortium (2021) CMMID COVID-19 Working Group;. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 372(6538):eabg3055. doi: https://doi.org/10.1126/science.abg3055
Regev-Yochay G, Amit S, Bergwerk M et al (2021) Decreased infectivity following BNT162b2 vaccination: A prospective cohort study in Israel. Lancet Reg Health Eur 7:100150. https://doi.org/10.1016/j.lanepe.2021.100150
Mahallawi WH, Alsamiri AD, Dabbour AF et al (2021) Association of viral load in SARS-CoV-2 patients with age and gender. Front Med 8:608215. https://doi.org/10.3389/fmed.2021.608215
Bailly B, Guilpain L, Bouiller K et al (2022) BNT162b2 messenger RNA vaccination did not prevent an outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 Variant 501Y. V2 in an elderly nursing home but reduced transmission and disease severity. Clin Infect Dis 74(3):517–520. https://doi.org/10.1093/cid/ciab446
Alahmari AA, Khan AA, Elganainy A et al (2021) Epidemiological and clinical features of COVID-19 patients in Saudi Arabia. J Infect Public Health 14(4):437–443. https://doi.org/10.1016/j.jiph.2021.01.003
Pritchard E, Matthews PC, Stoesser N et al (2021) Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med 27(8):1370–1378. https://doi.org/10.1038/s41591-021-01410-w
Shastri J, Parikh S, Aggarwal V et al (2021) Severe SARS-CoV-2 breakthrough reinfection with Delta variant after recovery from breakthrough infection by Alpha variant in a fully vaccinated health worker. Front Med 8:737007. https://doi.org/10.3389/fmed.2021.737007
Mlcochova P, Kemp SA, Dhar MS et al (2021) Indian SARS-CoV-2 Genomics Consortium (INSACOG); Genotype to Phenotype Japan (G2P-Japan) Consortium; CITIID-NIHR BioResource COVID-19 Collaboration; SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 599(7883):114–119. https://doi.org/10.1038/s41586-021-03944-y
Richterman A, Meyerowitz EA, Cevik M (2022) Indirect protection by reducing transmission: ending the pandemic with Severe Acute Respiratory Syndrome Coronavirus 2 vaccination. Open Forum Infect Dis 9(2):ofab259. https://doi.org/10.1093/ofid/ofab259
Levine-Tiefenbrun M, Yelin I, Katz R et al (2021) Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med 27:790–792. https://doi.org/10.1038/s41591-021-01316-7
Marks M, Millat-Martinez P, Ouchi D et al (2021) Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis 21(5):629–636. https://doi.org/10.1016/S1473-3099(20)30985-3
Jeffery-Smith A, Iyanger N, Williams SV et al (2021) Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro Surveill 26(5):2100092. https://doi.org/10.2807/1560-7917.ES.2021.26.5.2100092
Jawade K, Sinha AY, Bhagat S et al (2021) A novel ORF1a-based SARS-CoV-2 RT-PCR assay to resolve inconclusive samples. Int J Infect Dis 106:395–400. https://doi.org/10.1016/j.ijid.2021.04.006
Pande S, Bhanothu V, Mayekar A et al (2022) An approach to resolve uncertainty in COVID-19 diagnosis due to inconclusive results from RT-PCR test. J Assoc Physicians India 70(6):11–12 PMID: 35702857
Voysey M, Costa Clemens SA, Madhi SA et al (2021) Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 397(10277):881–891. https://doi.org/10.1016/S0140-6736(21)00432-3. Erratum in: Lancet. 397(10277):880
Yelin I, Katz R, Herzel E et al (2021) Associations of the BNT162b2 COVID-19 vaccine effectiveness with patient age and comorbidities. https://doi.org/10.1101/2021.03.16.21253686. medRxiv.2021.03.16.21253686
Hyams C, Marlow R, Maseko Z et al (2021) Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis 21(11):1539–1548. https://doi.org/10.1016/S1473-3099(21)00330-3
Harder T, Koch J, Vygen-Bonnet S et al (2021) Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Eurosurveillance 26(28):2100563. https://doi.org/10.2807/1560-7917.ES.2021.26.28.2100563
Tartof SY, Slezak JM, Fischer H et al (2021) Six-month effectiveness of BNT162B2 mRNA COVID-19 vaccine in a large US integrated health system: A retrospective cohort study [Internet]. Rochester, NY: Social Science Research Network; 2021 Aug [cited 2021 Sep 15]. Report No.: ID 3909743. Available from: https://papers.ssrn.com/abstract=3909743
Nordström P, Ballin M, Nordström A (2021) Effectiveness of Covid-19 vaccination against risk of symptomatic infection, hospitalization, and death up to 9 months: A Swedish total-population cohort study [Internet]. Rochester, NY: Social Science Research Network; 2021 Oct [cited 2021 Nov 17]. Report No.: ID 3949410. Available from: https://papers.ssrn.com/abstract=3949410
Pegu A, O’Connell SE, Schmidt SD et al (2021) Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 373(6561):1372–1377. https://doi.org/10.1126/science.abj4176
Mukherjee S, Pahan K (2021) Is COVID-19 Gender-sensitive? J Neuroimmune Pharmacol 16(1):38–47. https://doi.org/10.1007/s11481-020-09974-z
Gadi N, Wu SC, Spihlman AP, Moulton VR (2020) What’s sex got to do with COVID-19? Gender-based differences in the host immune response to coronaviruses. Front Immunol 11:2147. https://doi.org/10.3389/fimmu.2020
La Vignera S, Cannarella R, Condorelli RA et al (2020) Sex-specific SARS-CoV-2 mortality: among hormone-modulated ACE2 expression, risk of Venous Thromboembolism and Hypovitaminosis D. Int J Mol Sci 21(8):2948. https://doi.org/10.3390/ijms21082948
Zhao Y, Zhao Z, Wang Y et al (2020) Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med 202(5):756–759. https://doi.org/10.1164/rccm.202001-0179LE Erratum in: Am J Respir Crit Care Med. 2021;203(6):782.
Takahashi T, Ellingson MK, Wong P et al (2020) Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 588(7837):315–320. https://doi.org/10.1038/s41586-020-2700-3
Day M (2021) Covid-19: stronger warnings are needed to curb socialising after vaccination, say doctors. BMJ 372:n783. https://doi.org/10.1136/bmj.n783
Moore S, Hill EM, Tildesley MJ et al (2021) Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis 21(6):793–802. https://doi.org/10.1016/S1473-3099(21)00143-2
Chia WN, Zhu F, Ong SWX et al (2021) Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe 2(6):e240–e249. https://doi.org/10.1016/S2666-5247(21)00025-2
Dagan N, Barda N, Kepten E et al (2021) BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384(15):1412–1423. https://doi.org/10.1056/NEJMoa2101765
Cherian S, Potdar V, Jadhav S et al (2021) SARS-CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. Microorganisms 9(7):1542. https://doi.org/10.3390/microorganisms9071542
Garcia-Beltran WF, St Denis KJ, Hoelzemer A et al (2022) mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185(3):457–466e4. https://doi.org/10.1016/j.cell.2021.12.033
Wang P, Nair MS, Liu L et al (2021) Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 593(7857):130–135. https://doi.org/10.1038/s41586-021-03398-2
Muik A, Lui BG, Wallisch AK et al (2022) Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 375(6581):678–680. https://doi.org/10.1126/science.abn7591
Focosi D, Maggi F (2021) Neutralising antibody escape of SARS-CoV-2 spike protein: Risk assessment for antibody-based Covid-19 therapeutics and vaccines. Rev Med Virol 31(6):e2231. https://doi.org/10.1002/rmv.2231
Mueller AL, McNamara MS, Sinclair DA (2020) Why does COVID-19 disproportionately affect older people? Aging 12(10):9959–9981. https://doi.org/10.18632/aging.103344
Edara VV, Norwood C, Floyd K et al (2021) Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 29(4):516–521e3. https://doi.org/10.1016/j.chom.2021.03.009
Acknowledgment
This research article has been reviewed and edited to improve clarity and address language usage issues by Dr. Vainav Patel, a co-author of this manuscript (VP) and a native speaker of the English language.
We acknowledge the encouragement and support from the Indian Council of Medical Research (ICMR), the Ministry for Health & Family Welfare, the Government of India, and all the NIRRCH COVID-19 testing and reporting team, Mumbai, India.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflicts of interest exist.
Ethical approval
The study was approved by the Institutional Ethics Committee of ICMR-NIRRCH (Letter No# D/ICEC/Sci-199/214/2021).
Additional information
Communicated by Pablo Pineyro
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material
Supplementary Fig. S1:
(a) RT-PCR outcomes of COVID-19 suspected cases with an increase in the interval between partial vaccination and the RT-PCR test. The RT-PCR outcomes of COVID-19 suspected cases were represented as a percentage (%). (b) RT-PCR outcomes of COVID-19 suspected cases with an increase in the duration between complete vaccination and the RT-PCR test. The RT-PCR outcomes of COVID-19 suspected cases were represented as a percentage (%).
Supplementary Fig. S2:
(a) Dynamics of age (in years) in COVID-19 suspected cases with an increase in the duration between partial vaccination and the RT-PCR test. The data were represented as mean ± SD in years. (b) Dynamics of age (in years) in COVID-19 suspected cases with an increase in the duration between complete vaccination and the RT-PCR test. The data were represented as mean ± SD in years
Supplementary Fig. S3
: (a) Dynamics of Ct values of SARS-CoV-2 genes (ORF1a/b/N2 gene and E/N gene) with an increase in the duration between partial vaccination and the RT-PCR test. The data were represented as mean ± SD. (b) Dynamics of Ct values of SARS-CoV-2 genes (ORF1a/b/N2 gene and E/N gene) with an increase in the duration between full vaccination and the RT-PCR test. The data were represented as mean ± SD
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhanothu, V., Munne, K., Pande, S. et al. The dynamics of SARS-CoV-2 infection in unvaccinated and vaccinated populations in Mumbai, India, between 28 December 2020 and 30 August 2021. Arch Virol 168, 188 (2023). https://doi.org/10.1007/s00705-023-05815-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-023-05815-5