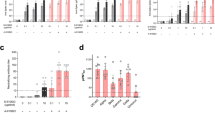
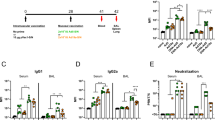
Abstract
Due to the rapid development of new variants of SARS-CoV-2 as well as the real threat of new coronavirus zoonosis events, the development of a preventive vaccine with a broader scope of functionality is highly desirable. Previously, we reported the functionality of a nasal formulation containing the nucleocapsid protein and the receptor-binding domain (RBD) of the spike protein of the Delta variant of SARS-CoV-2 combined with the ODN-39M adjuvant. This combination induced cross-reactive immunity in mucosal and systemic compartments at the sarbecovirus level. In the present study, we explored the magnitude of the immunity generated in BALB/c mice by the same formulation with alum added as an additional adjuvant, to enhance the humoral immunity against the two antigens. Animals were immunized with three doses of the bivalent formulation, administered by subcutaneous route. Humoral immunity was tested by ELISA, and the neutralizing capacity of the resulting antibodies (Abs) was evaluated using a surrogate test and a vesicular stomatitis virus (VSV) pseudovirus-based assay. Cell-mediated immunity was also investigated using an IFN-γ ELISpot assay. High levels of antibodies against both antigens (N and RBD) were obtained upon immunization. Anti-RBD Abs with neutralizing capacity reacted with the RBD of three SARS-CoV-2 variants tested, including Omicron. Abs recognizing the nucleocapsid proteins of SARS-CoV-1 and the SARS-CoV-2 Delta and Omicron variants were also detected. Taken together, these results suggest that this bivalent formulation could be an attractive component of a pancorona vaccine able to broaden the scope of humoral immunity against both antigens. This will be particularly important for the reinforcement of immunity in previously vaccinated and/or infected populations.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Thakur V, Bhola S, Thakur P, Patel SKS, Kulshrestha S, Ratho RK, Kumar P (2022) Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe. Infection 50:309–325. https://doi.org/10.1007/s15010-021-01734-2
Swerdlow DL, Finelli L (2020) Preparation for possible sustained transmission of 2019 novel coronavirus: lessons from previous epidemics. JAMA 323:1129–1130. https://doi.org/10.1001/jama.2020.1960
Rubin R (2021) The search for a single vaccine against coronaviruses yet to come. JAMA 326(2):118–120. https://doi.org/10.1001/jama.2021.9477
Morens DM, Taubenberger JK, Fauci AS (2022) Universal coronavirus vaccines—an urgent need. N Engl J Med 386:297–299. https://doi.org/10.1056/NEJMp2118468
Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell AM, Simons D, Blomquist PB, Zaidi A, Nash S, Iwani Binti Abdul Aziz N, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J (2022) Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 386:1532–1546. https://doi.org/10.1056/NEJMoa2119451
Dutta NK, Mazumdar K, Gordy JT (2020) The nucleocapsid protein of SARS–CoV-2: a target for vaccine development. J Virol 94:e00647-e720. https://doi.org/10.1128/JVI.00647-20
Matchett WE, Joag V, Stolley JM, Shepherd FK, Quarnstrom CF, Mickelson CK, Wijeyesinghe S, Soerens AG, Becker S, Thiede JM, Weyu E, O’Flanagan SD, Walter JA, Vu MN, Menachery VD, Bold TD, Vezys V, Jenkins MK, Langlois RA, Masopust D (2021) Cutting edge: nucleocapsid vaccine elicits spike-independent SARS-CoV-2 protective immunity. JI 207:376–379. https://doi.org/10.4049/jimmunol.2100421
Dangi T, Class J, Palacio N, Richner JM, Penaloza MacMaster P (2021) Combining spike- and nucleocapsid-based vaccines improves distal control of SARS-CoV-2. Cell Rep 36:109664. https://doi.org/10.1016/j.celrep.2021.109664
Phatarphekar A, Vidyadhar Reddy GEC, Gokhale A, Karanam G, Kuchroo P, Shinde K, Masand G, Pagare S, Khadpe N, Pai SS, Vijayan V, Ramnath RL, Pratap Reddy K, Rao P, Harinarayana Rao S, Ramana V (2022) RelCoVax®, a two antigen subunit protein vaccine candidate against SARS-CoV-2 induces strong immune responses in mice. Vaccine 40:4522–4530. https://doi.org/10.1016/j.vaccine.2022.06.026
Lobaina Y, Chen R, Suzarte E, Ai P, Huerta V, Musacchio A, Silva R, Tan C, Martin A, Lazo L, Guillén G, Yang K, Perera Y, Hermida L (2022) The nucleocapsid protein of SARS-CoV-2, combined with ODN-39M, is a potential component for an intranasal bivalent pancorona vaccine (preprint). Immunology. https://doi.org/10.1101/2022.06.02.494502
Gil L, Marcos E, Izquierdo A, Lazo L, Valdés I, Ambala P, Ochola L, Hitler R, Suzarte E, Álvarez M, Kimiti P, Ndung’u J, Kariuki T, Guzmán MG, Guillén G, Hermida L (2015) The protein DIIIC-2, aggregated with a specific oligodeoxynucleotide and adjuvanted in alum, protects mice and monkeys against DENV-2. Immunol Cell Biol 93:57–66. https://doi.org/10.1038/icb.2014.63
Lobaina Y, Trujillo H, García D, Gambe A, Chacon Y, Blanco A, Aguilar JC (2010) The effect of the parenteral route of administration on the immune response to simultaneous nasal and parenteral immunizations using a new HBV therapeutic vaccine candidate. Viral Immunol 23:521–529. https://doi.org/10.1089/vim.2010.0024
Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, Zhang L, Nie L, Qin H, Wang M, Lu Q, Li X, Sun Q, Liu J, Fan C, Huang W, Xu M, Wang Y (2020) Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat Protoc 15:3699–3715. https://doi.org/10.1038/s41596-020-0394-5
Scheiermann J, Klinman DM, Klinman D (2014) Vaccines targeting infectious diseases and cancer. Exp Rev Vaccines 32(48):6377–6389. https://doi.org/10.1016/j.vaccine.2014.06.065
Olivera S, Pérez A, Falcon V, Urquiza D, Pichardo D, Martínez-Donato G (2020) Protective cellular immune response against hepatitis C virus elicited by chimeric protein formulations in BALB/c mice. Adv Virol 165:593–607. https://doi.org/10.1007/s00705-019-04464-x
Aguilar JC, Lobaina Y, Muzio V, Garcia D, Penton E, Iglesias E, Pichardo D, Urquiza D, Rodriguez D, Silva D, Petrovsky N, Guillen G (2004) Development of a nasal vaccine for chronic hepatitis B infection that uses the ability of hepatitis B core antigen to stimulate a strong Th1 response against hepatitis B surface antigen. Immunol Cell Biol 82:539–546. https://doi.org/10.1111/j.1440-1711.2004.01278.x
Hong S-H, Oh H, Park YW, Kwak HW, Oh EY, Hyo-Jung P, Kang KW, Kim G, Koo B-S, Hwang E-H, Baek SH, Hyeong-Jun P, Lee Y-S, Bang Y-J, Kim J-Y, Bae S-H, Lee SJ, Seo K-W, Hak K, Kwon T, Kim J-H, Lee S, Kim E, Kim Y, Park J-H, Park S-I, Gonçalves M, Weon BM, Jeong H, Nam KT, Hwang K-A, Kim J, Hun K, Lee S-M, Hong JJ, Nam J-H (2021) Immunization with RBD-P2 and N protects against SARS-CoV-2 in nonhuman primates. Sci Adv 7:7156. https://doi.org/10.1126/sciadv.abg7156
Matchett WE, Joag JV, Stolley M, Shepherd FK, Quarnstrom CK, Mickelson SW, Soerens AG, Becker S, Thiede JM, Weyu E, O’Flanagan SD, Walter JA, Vu MN, Vineet D, Menachery TD, Bold VV, Jenkins MK, Langlois RA, Masopust D (2021) Cutting Edge: Nucleocapsid vaccine elicits spike-independent SARS-CoV-2 protective immunity. J Immunol 207:376–379. https://doi.org/10.4049/jimmunol.2100421
Harris PE, Brasel T, Massey C, Herst CV, Burkholz S, Lloyd P, Blankenberg T, Bey TM, Carback R, Hodge T, Ciotlos S, Wang L, Comer JE, Rubsamen RM (2021) A synthetic peptide CTL vaccine targeting nucleocapsid confers protection from SARS-CoV-2 challenge in rhesus macaques. Vaccines 9(5):520. https://doi.org/10.3390/vaccines9050520
Zhuang Z, Lai X, Sun JJ, Chen Z, Zhang Z, Dai J, Liu D, Li Y, Li F, Wang Y, Zhu A, Wang J, Yang W, Huang J, Li X, Hu L, Wen L, Zhuo J, Zhang Y, Chen D, Li S, Huang S, Yongxia S, Zheng K, Nanshan Z, Jingxian Z, Zhou D, Zhao JJ (2021) Mapping and role of T cell response in SARS-CoV-2–infected mice. J Exp Med 218(4):e20202187. https://doi.org/10.1084/jem.20202187
Wang Q, Zhang L, Kuwahara K, Li L, Liu Z, Li T, Zhu H, Liu J, Xu Y, Xie J, Morioka H, Sakaguchi N, Qin C, Liu G (2016) Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2(5):361–376. https://doi.org/10.1021/acsinfecdis.6b00006
Agrawal AS, Tao X, Algaissi A, Garron T, Narayanan T, Peng B-H, Couch RB, Tseng C-TK (2016) Immunization with inactivated Middle East respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother 12(9):2351–2356. https://doi.org/10.1080/21645515.2016.1177688
Li X, Huang Y, Wang W, Jing GL, Zhang CH, Qin PZ, Guan WJ, Gan L, Li YL, Liu WH, Dong H, Miao YT, Fan SJ, Zhang ZB, Zhang DM, Zhong NS (2021) Effectiveness of inactivated SARSCoV- 2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real world study. Emerg Microbes Infect 10(1):1751–1759. https://doi.org/10.1080/22221751.2021.1969291
Li D, Luan N, Li J, Zhao H, Zhang Y, Long R, Jiang G, Fan S, Xu X, Cao H, Wang Y, Liao Y, Wang L, Liu L, Liu C, Li Q (2021) Waning antibodies from inactivated SARS-CoV-2 vaccination offer protection against infection without antibody-enhanced immunopathology in rhesus macaque pneumonia models. Emerg Microbes Infect 10(1):2194–2198. https://doi.org/10.1080/22221751.2021.2002670
Hernández-Bernal F, Ricardo-Cobas MC, Martín-Bauta Y, Navarro-Rodríguez Z, Piñera-Martínez M, Quintana-Guerra J, Urrutia-Pérez K, Chávez-Chong CO, Azor-Hernández JL, Rodríguez-Reinoso JL, Lobaina-Lambert L, Colina-Ávila E, Bizet-Almeida J, Rodríguez-Nuviola J, del Valle-Piñera S, Ramírez-Domínguez M, Tablada-Ferreiro E, Alonso-Valdés M, Lemos-Pérez G, Guillén-Nieto GE, Palenzuela-Díaz A, Noa-Romero E, Limonta-Fernández M, Fernández-Ávila JM, Ali-Mros NA, del Toro-Lahera L, Remedios-Reyes R, Ayala-Ávila M, Muzio-González VL (2022) Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: a randomised, double-blind, placebo-controlled, phase 1–2 clinical trial (ABDALA Study). eClinicalMedicine 46:101383. https://doi.org/10.1016/j.eclinm.2022.101383
Pérez-Rodríguez S, Rodríguez-González MC, Ochoa-Azze R, Climent-Ruiz Y, González-Delgado CA, Paredes-Moreno V-S, Rodríguez-Noda L, Perez-Nicado R, González-Mugica R, Martínez-Pérez M, Sánchez-Ramírez B, Hernández-García T, Díaz-Machado A, Tamayo-Rodríguez M, Martín-Trujillo A, Rubino-Moreno J, Suárez-Batista A, Dubed-Echevarría M, Pérez-Guevara MT, Amoroto-Roig M, Chappi-Estévez Y, Bergado-Báez G, Pi-Estopiñán F, Chen GW, Valdés-Balbín Y, García-Rivera D, Verez-Bencomo V (2022) A randomized, double-blind phase I clinical trial of two recombinant dimeric RBD COVID-19 vaccine candidates: safety, reactogenicity and immunogenicity. Vaccine 40:2068–2075. https://doi.org/10.1016/j.vaccine.2022.02.029
Yang S, Li Y, Dai L, Wang J, He P, Li C, Fang X, Wang C, Zhao X, Huang E, Wu C, Zhong Z, Wang F, Duan X, Tian S, Wu L, Liu Y, Luo Y, Chen Z, Li F, Li J, Yu X, Ren H, Liu L, Meng S, Yan J, Hu Z, Lidong Gao L, Gao GF (2021) Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis 21(8):P1107–P1119. https://doi.org/10.1016/S1473-3099(21)00127-4
Dangi T, Sanchez S, Park M, Class J, Richner JM, Penaloza-MacMaster P (2022) Nucleocapsid-specific humoral responses improve the control of SARS-CoV-2 (preprint). Immunology. https://doi.org/10.1101/2022.03.09.483635
Herman JD, Wuang C, Burke JS, Zur Y, Compere H, Kang J, Macvicar R, Shin S, Frank I, Siegel D, Tebas P, Choi GH, Shaw PA, Yoon H, Liise-annePirofski L-A, Juelg B, Bar KJ, Lauffenburger D, Alter G (2022) A role for nucleocapsid-specific antibody function in Covid-19. Convalescent Plasma Ther (Preprint). https://doi.org/10.1101/2022.02.19.22271230
Caddy SL, Vaysburd M, Papa G, Wing M, O’Connell K, Stoycheva D, Foss S, Terje Andersen J, Oxenius A, James LC (2021) Viral nucleoprotein antibodies activate TRIM21 and induce T cell immunity. EMBO J. https://doi.org/10.15252/embj.202010622
Tilocca B, Soggiu A, Sanguinetti M, Musella V, Britti D, Bonizzi L, Urbani A, Roncada P (2020) Comparative computational analysis of SARS-CoV-2 nucleocapsid protein epitopes in taxonomically related coronaviruses. Microbes Infect 22:188–194. https://doi.org/10.1016/j.micinf.2020.04.002
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP (2021) Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27:1205–1211. https://doi.org/10.1038/s41591-021-01377-8
Zhu DY, Gorman MJ, Yuan D, Yu J, Mercado NB, McMahan K, Borducchi EN, Lifton M, Liu J, Nampanya F, Patel S, Peter L, Tostanoski LH, Pessaint L, Ry AV, Finneyfrock B, Velasco J, Teow E, Brown R, Cook A, Andersen H, Lewis MG, Lauffenburger DA, Barouch DH, Alter G (2022) Defining the determinants of protection against SARS-CoV-2 infection and viral control in a dose-down Ad26.CoV2.S vaccine study in nonhuman primates. PLOS Biol 20:e3001609. https://doi.org/10.1371/journal.pbio.3001609
Kleanthous H, Silverman JM, Makar KW, Yoon I-K, Jackson N, Vaughn DW (2021) Scientific rationale for developing potent RBD-based vaccines targeting COVID-19. npj Vaccines 6:128. https://doi.org/10.1038/s41541-021-00393-6
Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Morillas Ramos G, Dopfer-Jablonka A, Heidemann A, Ritter C, Friedrichsen M, Schultze-Florey C, Ravens I, Willenzon S, Bubke A, Ristenpart J, Janssen A, Ssebyatika G, Bernhardt G, Münch J, Hoffmann M, Pöhlmann S, Krey T, Bošnjak B, Förster R, Behrens MN (2021) Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1nCoV-19/BNT162b2 vaccination. Nat Med 27:1525–1529. https://doi.org/10.1038/s41591-021-01449-9
Lee B, Ko J-H, Park J, Moon H-W, Baek JY, Jung S, Lim H-Y, Kim K-C, Huh K, Cho SY, Kang C-I, Chung DR, Huh HJ, Chung CR, Kim Y-J, Joo E-J, Kang E-S, Peck KR (2022) Estimating the neutralizing effect and titer correlation of semi-quantitative anti-SARS-CoV-2 antibody immunoassays. Front Cell Infect Microbiol 12:822599. https://doi.org/10.3389/fcimb.2022.822599
Tan CW, Chia WN, Qin X, Liu P, Chen MI-C, Tiu C, Hu Z, Chih-Wei Chen V, Young BE, Sia WR, Tan Y-J, Foo R, Yi Y, Lye DC, Anderson DE, Wang L-F (2020) A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol 38:1073–1078. https://doi.org/10.1038/s41587-020-0631-z
Yuan M, Wu NC, Zhu X, Lee CC, So R, Lv H, Mok C, Wilson IA (2020) A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science 368(6491):630–633. https://doi.org/10.1126/science.abb7269
Makitalo B, Lundholm P, Hinkula J, Nilsson C, Karlen K, Morner A, Sutter G, Erfle V, Heeney JL, Wahren B, Biberfeld G, Thorstensson R (2004) Enhanced cellular immunity and systemic control of SHIV infection by combined parenteral and mucosal administration of a DNA prime MVA boost vaccine regimen. J Gen Virol 85:2407–2419. https://doi.org/10.1099/vir.0.79869-0
Acknowledgements
This work was supported by MOST “National Key R&D Program of China (2021YFE0192200)”, “PNCT CITMA, Cuba”, “Hunan Provincial Base for Scientific and Technological Innovation Cooperation (2019CB1012)”, “The Science and Technology Innovation Program of Hunan Province, (2020RC5035)”, and “Hunan Provincial Innovative Construction Program (2020WK2031)”.
Author information
Authors and Affiliations
Contributions
YL: conceptualization, investigation, formal analysis, writing—original draft. RC: investigation. ES: conceptualization, formal analysis. PA: investigation, resources. VH: investigation, formal analysis. CT: investigation, resources. LA-L: formal analysis. YL: investigation. AM: investigation. RS: supervision. GG: supervision. JZ: supervision. KY: project administration. YP: conceptualization, supervision, formal analysis, project administration. LH: conceptualization, supervision, funding acquisition, formal analysis, writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee of Beijing Vital River Laboratory Animal Technology Co., Ltd. The standards of the laboratory animal room complied with the national standards of the People's Republic of China GB14925-2010. The human sera from COVID-19 convalescent and negative individuals were collected at The Eighth and Ninth People’s Hospital of the city of Dongguan (Guangdong Province, China). The study protocol was approved by the institutional ethics committees of both hospitals and was carried out in accordance with the principles of the Helsinki declaration.
Additional information
Handling Editor: John Ziebuhr.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lobaina, Y., Chen, R., Suzarte, E. et al. Broad humoral immunity generated in mice by a formulation composed of two antigens from the Delta variant of SARS-CoV-2. Arch Virol 168, 190 (2023). https://doi.org/10.1007/s00705-023-05812-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-023-05812-8