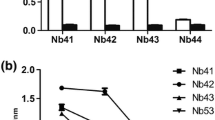
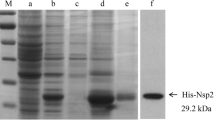
Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) causes porcine reproductive and respiratory syndrome (PRRS) worldwide, especially in domestic pigs, with an enormous economic impact, estimated at $664 million in losses every year to the pig industry. Current vaccines confer limited protection, and no direct-acting anti-PRRS treatment is available. Non-structural protein (NSP) 1β, a cysteine-like protease (CLPro) of PRRSV plays an essential role in viral polyprotein processing, subgenomic RNA synthesis, and evasion of host innate immunity. Therefore, agents that interfere with the bioactivity of NSP1β would be expected to inhibit virus replication. In this study, a porcine single-chain antibody (scFv)-phage display library was constructed and used as a tool for production of NSP1β-specific porcine scFvs (pscFvs). The pscFvs to NSP1β were linked to a cell-penetrating peptide to form cell-penetrating pscFvs (transbodies), which could be internalized and inhibit PRRSV replication in infected cells. A computer simulation indicated that the effective pscFvs used several residues in multiple complementarity determining regions (CDRs) to interact with multiple residues in the CLPro and C-terminal motifs, which might explain the mechanism of pscFv-mediated inhibition of virus replication. Although experiments are needed to determine the antiviral mechanism of the transbodies, the current data indicate that transbodies can potentially be applied for treatment and prevention of PRRSV infection.






Similar content being viewed by others
Data Availability
Data is available.
References
Polson DD, Marsh WE, Dial GD (1992) Financial evaluation and decision making in the swine breeding herd. Vet Clin N Am Food Anim Pract 8(3):725–747
Christianson WT, Choi CS, Collins JE, Molitor TW, Morrison RB, Joo HS (1993) Pathogenesis of porcine reproductive and respiratory syndrome virus infection in mid-gestation sows and fetuses. Can J Vet Res 57(4):262–268
Rossow KD, Collins JE, Goyal SM, Nelson EA, Christopher-Hennings J, Benfield DA (1995) Pathogenesis of porcine reproductive and respiratory syndrome virus infection in gnotobiotic pigs. Vet Pathol 32(4):361–373
Rowland RR, Lunney J, Dekkers J (2012) Control of porcine reproductive and respiratory syndrome (PRRS) through genetic improvements in disease resistance and tolerance. Front Genet 3:260. https://doi.org/10.3389/fgene.2012.00260
Brockmeier SL, Loving CL, Vorwald AC, Kehrli ME Jr, Baker RB, Nicholson TL, Lager KM, Miller LC, Faaberg KS (2012) Genomic sequence and virulence comparison of four Type 2 porcine reproductive and respiratory syndrome virus strains. Virus Res 169(1):212–221
Meulenberg JJ, van Nieuwstadt AP, van Essen-Zandbergen A, Bos-de Ruijter JN, Langeveld JP, Meloen RH (1998) Localization and fine mapping of antigenic sites on the nucleocapsid protein N of porcine reproductive and respiratory syndrome virus with monoclonal antibodies. Virology 252(1):106–114
Snijder EJ, Kikkert M, Fang Y (2013) Arterivirus molecular biology and pathogenesis. J Gen Virol 94(Pt 10):2141–2163
Meng XJ, Paul PS, Halbur PG, Lum MA (1995) Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the U.S.A. and Europe. Arch Virol. 140(4):745–755
Beura LK, Sarkar SN, Kwon B, Subramaniam S, Jones C, Pattnaik AK, Osorio FA (2010) Porcine reproductive and respiratory syndrome virus nonstructural protein 1beta modulates host innate immune response by antagonizing IRF3 activation. J Virol 84(3):1574–1584
den Boon JA, Faaberg KS, Meulenberg JJ, Wassenaar AL, Plagemann PG, Gorbalenya AE, Snijder EJ (1995) Processing and evolution of the N-terminal region of the arterivirus replicase ORF1a protein: identification of two papain-like cysteine proteases. J Virol 69(7):4500–4505
Fang Y, Snijder EJ (2010) The PRRSV replicase: exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res 154(1–2):61–76
Song C, Krell P, Yoo D (2010) Nonstructural protein 1α subunit-based inhibition of NF-κB activation and suppression of interferon-β production by porcine reproductive and respiratory syndrome virus. Virology 407(2):268–280
Jantafong T, Boonsoongnern A, Poolperm P, Urairong K, Lekcharoensuk C, Lekcharoensuk P (2011) Genetic characterization of porcine circovirus type 2 in piglets from PMWS-affected and -negative farms in Thailand. Virol J. 8:88
Thanongsaksrikul J, Srimanote P, Tongtawe P, Glab-Ampai K, Malik AA, Supasorn O, Chiawwit P, Poovorawan Y, Chaicumpa W (2018) Identification and production of mouse scFv to specific epitope of enterovirus-71 virion protein-2 (VP2). Arch Virol 163(5):1141–1152
Kulkeaw K, Sakolvaree Y, Srimanote P, Tongtawe P, Maneewatch S, Sookrung N, Tungtrongchitr A, Tapchaisri P, Kurazono H, Chaicumpa W (2009) Human monoclonal ScFv neutralize lethal Thai cobra, Naja kaouthia, neurotoxin. J Proteomics 72(2):270–282
Derossi D, Joliot AH, Chassaing G, Prochiantz A (1994) The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem 269(14):10444–10450
Thueng-in K, Thanongsaksrikul J, Srimanote P, Bangphoomi K, Poungpair O, Maneewatch S, Choowongkomon K, Chaicumpa W (2012) Cell penetrable humanized-VH/V(H)H that inhibit RNA dependent RNA polymerase (NS5B) of HCV. PLoS ONE 7(11):e49254
Elmar Krieger, Molecular graphics, modeling and simulation program for Linux, Windows and MacOS. http://www.yasara.org
Xue F, Sun Y, Yan L, Zhao C, Chen J, Bartlam M, Li X, Lou Z, Rao ZJ (2010) The crystal structure of porcine reproductive and respiratory syndrome virus nonstructural protein Nsp1beta reveals a novel metal-dependent nuclease. Virol 84(13):6461–6471
Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y (2015) The I-TASSER Suite: protein structure and function prediction. Nat Methods 12(1):7–8
Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256(5517):495–497
Norman DJ, Shield CF 3rd, Barry JM, Henell K, Funnell MB, Lemon J (1987) Therapeutic use of OKT3 monoclonal antibody for acute renal allograft rejection. Nephron 46(Suppl 1):41–47
Nelson AL, Dhimolea E, Reichert JM (2010) Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 9(10):767–774
Osbourn J, Groves M, Vaughan T (2005) From rodent reagents to human therapeutics using antibody guided selection. Methods 36(1):61–68
Agrawal AG, Petersen LR (2003) Human immunoglobulin as a treatment for West Nile virus infection. J Infect Dis 188(1):1–4
Rasetti-Escargueil C, Avril A, Chahboun S, Tierney R, Bak N et al (2015) Development of human-like scFv-Fc antibodies neutralizing Botulinum toxin serotype B. MAbs 7(6):1161–1177
Wang H, Yu R, Fang T, Yu T, Chi X et al (2016) Tetanus neurotoxin neutralizing antibodies screened from a human immune scFv antibody phage display library. Toxins 8(9):266
Lee CH, Lee YC, Lee YL et al (2017) Single chain antibody fragment against venom from the snake Daboia russelii formosensis. Toxins (Basel). 9(11):347
Sapats SI, Trinidad L, Gould G, Heine HG, Van Den Berg TP, Eterradossi N, Jackwood D, Parede L, Toquin D, Ignjatovic J (2006) Chicken recombinant antibodies specific for very virulent infectious bursal disease virus. Arch Virol 151:1551–1566
Braganza A, Wallace K, Pell L, Parrish CR, Siegel DL, Mason NJ (2011) Generation and validation of canine single chain variable fragment phage display libraries. Vet Immunol Immunopathol 139:27–40
Zhang F, Chen Y, Yang L, Zhu J (2019) Construction and characterization of porcine single-chain fragment variable antibodies that neutralize transmissible gastroenteritis virus in vitro. Arch Virol 164:983–994
Li F, Aitken R (2004) Cloning of porcine scFv antibodies by phage display and expression in Escherichia coli. Vet Immunol Immunopathol 97(1–2):39–51
Kroese MV, Zevenhoven-Dobbe JC, Bos-de Ruijter JNA, Peeters BPH, Meulenberg JJM, Cornelissen LAHM, Snijder EJ (2008) The nsp1alpha and nsp1 papain-like autoproteinases are essential for porcine reproductive and respiratory syndrome virus RNA synthesis. J Gen Virol 89(Pt 2):494–499
Sun Z, Chen Z, Lawson SR, Fang Y (2010) The cysteine protease domain of porcine reproductive and respiratory syndrome virus nonstructural protein 2 possesses deubiquitinating and interferon antagonism functions. J Virol 84(15):7832–7846
Farina GA, York MR, Di Marzio M et al (2010) Poly(I:C) drives type I IFN- and TGFβ-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol 130(11):2583–2593
Acknowledgements
This work was supported by the Thailand Research Fund, Office of the Higher Education Commission and Kasetsart University, Thailand (Grant number MRG5680053).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Handling Editor: Tim Skern.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thueng-in, K., Theerawatanasirikul, S., Meechan, P. et al. Cell-penetrating porcine single-chain antibodies (transbodies) against nonstructural protein 1β (NSP1β) of porcine reproductive and respiratory syndrome virus inhibit virus replication. Arch Virol 168, 133 (2023). https://doi.org/10.1007/s00705-023-05760-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-023-05760-3