Abstract
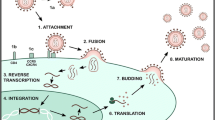
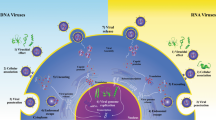
Arthropod-borne viruses (arboviruses), such as Zika virus (ZIKV), chikungunya virus (CHIKV), dengue virus (DENV), yellow fever virus (YFV), and West Nile virus (WNV), are pathogens of global importance. Therefore, there has been an increasing need for new drugs for the treatment of these viral infections. In this context, antimicrobial peptides (AMPs) obtained from animal venoms stand out as promising compounds because they exhibit strong antiviral activity against emerging arboviral pathogens. Thus, we systematically searched and critically analyzed in vitro and in vivo studies that evaluated the anti-arbovirus effect of peptide derivatives from toxins produced by vertebrates and invertebrates. Thirteen studies that evaluated the antiviral action of 10 peptides against arboviruses were included in this review. The peptides were derived from the venom of scorpions, spiders, wasps, snakes, sea snails, and frogs and were tested against DENV, ZIKV, YFV, WNV, and CHIKV. Despite the high structural variety of the peptides included in this study, their antiviral activity appears to be associated with the presence of positive charges, an excess of basic amino acids (mainly lysine), and a high isoelectric point (above 8). These peptides use different antiviral mechanisms, the most common of which is the inhibition of viral replication, release, entry, or fusion. Moreover, peptides with virucidal and cytoprotective (pre-treatment) effects were also identified. In conclusion, animal-venom-derived peptides stand out as a promising alternative in the search and development of prototype antivirals against arboviruses.


Similar content being viewed by others
References
Gould E, Pettersson J, Higgs S et al (2017) Emerging arboviruses: why today? One Health (Amsterdam, Netherlands) 4:1–13. https://doi.org/10.1016/j.onehlt.2017.06.001
Sukhralia S, Verma M, Gopirajan S et al (2018) From dengue to Zika: the wide spread of mosquito-borne arboviruses. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-018-3375-7
Grischott F, Puhan M, Hatz C, Schlagenhauf P (2016) Non-vector-borne transmission of Zika virus: a systematic review. Travel Med Infect Dis 14:313–330. https://doi.org/10.1016/j.tmaid.2016.07.002
Gubler DJ (2001) Human arbovirus infections worldwide. In: Annals of the New York Academy of Sciences. New York Academy of Sciences, pp 13–24
Organization WH (2009) Dengue: Guidelines for Diagnosis Treatment Prevention and Control (New Edition 2009). World Health Organization, Geneva
Bhatt S, Gething PW, Brady OJ et al (2013) The global distribution and burden of dengue. Nature 496:504–507. https://doi.org/10.1038/nature12060
Lima WG, Souza NA, Fernandes SOA et al (2019) Serum lipid profile as a predictor of dengue severity: a systematic review and meta-analysis. Rev Med Virol. https://doi.org/10.1002/rmv.2056
Godói IP, Lima WG, Comar M et al (2017) Docking and QM/MM studies of NS2B-NS3pro inhibitors: a molecular target against the dengue virus. J Braz Chem Soc 28:895–906. https://doi.org/10.21577/0103-5053.20160242
Leonel CA, Lima WG, dos Santos M et al (2018) Pharmacophoric characteristics of dengue virus NS2B/NS3pro inhibitors: a systematic review of the most promising compounds. Arch Virol 163:575–586. https://doi.org/10.1007/s00705-017-3641-5
Santos FRS, Nunes DAF, Lima WG et al (2020) Identification of Zika virus NS2B-NS3 protease inhibitors by structure-based virtual screening and drug repurposing approaches. J Chem Inf Model 60:731–737. https://doi.org/10.1021/acs.jcim.9b00933
Santos FRS, Lima WG, Maia EHB et al (2020) Identification of a potential Zika virus inhibitor targeting NS5 methyltransferase using virtual screening and molecular dynamics simulations. J Chem Inf Model 60:562–568. https://doi.org/10.1021/acs.jcim.9b00809
da Mata ÉCG, Mourão CBF, Rangel M, Schwartz EF (2017) Antiviral activity of animal venom peptides and related compounds. J Venom Anim Toxins Incl Trop Dis 23:1–12
El-Seedi H, Abd El-Wahed A, Yosri N et al (2020) Antimicrobial properties of Apis mellifera’s bee venom. Toxins (Basel) 12:451. https://doi.org/10.3390/toxins12070451
Perumal Samy R, Stiles BG, Franco OL et al (2017) Animal venoms as antimicrobial agents. Biochem Pharmacol 134:127–138
Primon-Barros M, José Macedo A (2017) Animal venom peptides: potential for new antimicrobial agents. Curr Top Med Chem 17:1119–1156. https://doi.org/10.2174/1568026616666160930151242
Suranse V, Srikanthan A, Sunagar K (2018) Animal venoms: origin, diversity and evolution. Wiley, Chichester
Utkin YN (2015) Animal venom studies: current benefits and future developments. World J Biol Chem 6:28. https://doi.org/10.4331/wjbc.v6.i2.28
Chen N, Xu S, Zhang Y, Wang F (2018) Animal protein toxins: origins and therapeutic applications. Biophys Reports 4:233–242. https://doi.org/10.1007/s41048-018-0067-x
de Lima ME, de Pimenta AMC, Martin-Eauclaire MF et al (2009) Animal toxins: state of the art—perspectives in health and biotechnology. J Venom Anim Toxins Incl Trop Dis 15:585–586. https://doi.org/10.1590/S1678-91992009000300021
Peigneur S, de Lima ME, Tytgat J (2018) Phoneutria nigriventer venom: a pharmacological treasure. Toxicon 151:96–110
Lima WG, de Brito JCM, Cardoso VN, Fernandes SOA (2021) In-depth characterization of antibacterial activity of melittin against Staphylococcus aureus and use in a model of non-surgical MRSA-infected skin wounds. Eur J Pharm Sci. https://doi.org/10.1016/j.ejps.2020.105592
Lima WG, Brito JCM, de Lima ME et al (2021) A short synthetic peptide, based on LyeTx I from Lycosa erythrognatha venom, shows potential to treat pneumonia caused by carbapenem-resistant Acinetobacter baumannii without detectable resistance. J Antibiot (Tokyo). https://doi.org/10.1038/s41429-021-00421-6
Lima WG, Brito JCM, da Cruz Nizer WS (2020) Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2). Phyther Res 35(2):743–750. https://doi.org/10.1002/ptr.6872
Uddin MB, Lee BH, Nikapitiya C et al (2016) Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J Microbiol 54:853–866. https://doi.org/10.1007/s12275-016-6376-1
Santos DM, Verly RM, Piló-Veloso D et al (2010) LyeTx I, a potent antimicrobial peptide from the venom of the spider Lycosa erythrognatha. Amino Acids 39:135–144. https://doi.org/10.1007/s00726-009-0385-x
Fuscaldi LL, de Avelar Júnior JT, dos Santos DM et al (2020) Shortened derivatives from native antimicrobial peptide LyeTx I: in vitro and in vivo biological activity assessment. Exp Biol Med. https://doi.org/10.1177/1535370220966963
Melo-Braga MN, De Marco AF, dos Santos DM et al (2020) Antimicrobial peptides from lycosidae (Sundevall, 1833) spiders. Curr Protein Pept Sci 21:527–541. https://doi.org/10.2174/1389203721666200116091911
Da SCN, Da SFR, Dourado LFN et al (2019) A new topical eye drop containing lyetxi-b, a synthetic peptide designed from a lycosa erithrognata venom toxin, was effective to treat resistant bacterial keratitis. Toxins (Basel). https://doi.org/10.3390/toxins11040203
Kang SJ, Park SJ, Mishig-Ochir T, Lee BJ (2014) Antimicrobial peptides: therapeutic potentials. Expert Rev Anti Infect Ther 12:1477–1486
Wang G (2020) The Antimicrobial Peptide Database (APD). In: Dep. Pathol. Microbiol. Univ. Nebraska Med. Cent. https://wangapd3.com/contact.php. Accessed 6 Jun 2021
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100. https://doi.org/10.1371/journal.pmed.1000100
Mendes Oliveira VR, Paiva MC, Lima WG (2019) Plasmid-mediated colistin resistance in Latin America and Caribbean: a systematic review. Travel Med Infect Dis. https://doi.org/10.1016/j.tmaid.2019.07.015
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Carballar-Lejarazú R, Rodríguez MH, De La Cruz H-H et al (2008) Recombinant scorpine: a multifunctional antimicrobial peptide with activity against different pathogens. Cell Mol Life Sci 65:3081–3092. https://doi.org/10.1007/s00018-008-8250-8
El-Bitar AMH, Sarhan M, Abdel-Rahman MA et al (2020) Smp76, a scorpine-like peptide isolated from the venom of the scorpion scorpio maurus palmatus, with a potent antiviral activity against hepatitis C virus and dengue virus. Int J Pept Res Ther 26:811–821. https://doi.org/10.1007/s10989-019-09888-2
Ji M, Zhu T, Xing M et al (2019) An antiviral peptide from Alopecosa nagpag spider targets NS2B–NS3 protease of flaviviruses. Toxins (Basel) 11:584. https://doi.org/10.3390/toxins11100584
Ji Z, Li F, Xia Z et al (2018) The scorpion venom peptide Smp76 inhibits viral infection by regulating type-I interferon response. Virol Sin 33:545–556. https://doi.org/10.1007/s12250-018-0068-4
Li F, Lang Y, Ji Z et al (2019) A scorpion venom peptide Ev37 restricts viral late entry by alkalizing acidic organelles. J Biol Chem 294:182–194. https://doi.org/10.1074/jbc.RA118.005015
Monteiro JMC, Oliveira MD, Dias RS et al (2018) The antimicrobial peptide HS-1 inhibits dengue virus infection. Virology 514:79–87. https://doi.org/10.1016/j.virol.2017.11.009
Rothan HA, Bahrani H, Rahman NA, Yusof R (2014) Identification of natural antimicrobial agents to treat dengue infection: In vitro analysis of latarcin peptide activity against dengue virus. BMC Microbiol. https://doi.org/10.1186/1471-2180-14-140
Rothan HA, Bahrani H, Shankar EM et al (2014) Inhibitory effects of a peptide-fusion protein (Latarcin-PAP1-Thanatin) against chikungunya virus. Antivir Res 108:173–180. https://doi.org/10.1016/j.antiviral.2014.05.019
Sample CJ, Hudak KE, Barefoot BE et al (2013) A mastoparan-derived peptide has broad-spectrum antiviral activity against enveloped viruses. Peptides 48:96–105. https://doi.org/10.1016/j.peptides.2013.07.014
Müller VDM (2011) Avaliação da atividade antiviral de peçonhas de serpentes e escorpião contra os vírus da dengue e da febre amarela. Faculdade de Ciências Farmacêuticas de Ribeirão Preto
Xing M, Ji M, Hu J et al (2020) Snake cathelicidin derived peptide inhibits Zika virus infection. Front Microbiol. https://doi.org/10.3389/fmicb.2020.01871
Xu S, Li H, Shao X et al (2012) Critical effect of peptide cyclization on the potency of peptide inhibitors against dengue virus NS2B-NS3 protease. J Med Chem 55:6881–6887. https://doi.org/10.1021/jm300655h
Santana CJC, Magalhães ACM, Prías-Márquez CA et al (2020) Biological properties of a novel multifunctional host defense peptide from the skin secretion of the chaco tree frog, boana raniceps. Biomolecules. https://doi.org/10.3390/biom10050790
Lee H, Halverson S, Ezinwa N (2018) Mosquito-borne diseases. Prim Care Clin Off Pract 45:393–407. https://doi.org/10.1016/j.pop.2018.05.001
Paixão ES, Teixeira MG, Rodrigues LC (2018) Zika, chikungunya and dengue: the causes and threats of new and reemerging arboviral diseases. BMJ Glob Health 3:e000530. https://doi.org/10.1136/bmjgh-2017-000530
Saez NJ, Senff S, Jensen JE et al (2010) Spider-venom peptides as therapeutics. Toxins (Basel) 2:2851–2871
Mulder KCL, Lima LA, Miranda VJ et al (2013) Current scenario of peptide-based drugs: the key roles of cationic antitumor and antiviral peptides. Front Microbiol 4:321. https://doi.org/10.3389/fmicb.2013.00321
Skalickova S, Heger Z, Krejcova L et al (2015) Perspective of use of antiviral peptides against influenza virus. Viruses 7:5428–5442
Godói IP, da Rocha Taranto MF, de Lima WG, Alves RJ, Comar Júnior M, Maria J, Ferreira S, Taranto AG (2014) NS2B-NS3pro as a molecular target drugs development against dengue. Biochem Biotechnol Rep 3(2):16–30. https://doi.org/10.5433/2316-52002014v3p2p16
Niyomrattanakit P, Winoyanuwattikun P, Chanprapaph S et al (2004) Identification of residues in the dengue virus type 2 NS2B cofactor that are critical for NS3 protease activation. J Virol 78:13708–13716. https://doi.org/10.1128/jvi.78.24.13708-13716.2004
Noble CG, Seh CC, Chao AT, Shi PY (2012) Ligand-bound structures of the dengue virus protease reveal the active conformation. J Virol 86:438–446. https://doi.org/10.1128/jvi.06225-11
Almaaytah A, Albalas Q (2014) Scorpion venom peptides with no disulfide bridges: a review. Peptides 51:35–45
Acknowledgments
W.G.L. and J.M.A are grateful to Coordenação de Aperfeiçoamento de Pessoal do Nível Superior (CAPES) for a Ph.D. fellowship. M.P.R is grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a DTI fellowship. M.E.L. is grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for grants.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the development, analysis, and drafting of this article.
Corresponding author
Ethics declarations
Conflict of interest
All authors report that they do not have any conflicts of interest.
Ethical approval
Not applicable.
Additional information
Handling Editor: Patricia Aguilar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lima, W.G., Maia, C.Q., de Carvalho, T.S. et al. Animal venoms as a source of antiviral peptides active against arboviruses: a systematic review. Arch Virol 167, 1763–1772 (2022). https://doi.org/10.1007/s00705-022-05494-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-022-05494-8