Abstract
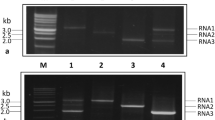
An outbreak in northwestern Turkey of prunus necrotic ringspot virus (PNRSV, genus Ilarvirus, family Bromoviridae) was sampled in 2016-2018. Gene sequences from these isolates, together with all of the gene sequence data for this virus in the GenBank database (>300 non-recombinant coat protein (CP) genes and 20 complete genomic sequences) were analysed to determine the relationship of the Turkish PNRSV isolates to those from other parts of the world. Phylogenetic and population genetic methods independently showed that the most recent common ancestor of the world PNRSV population was probably American, not Eurasian. PNRSV has spread to Turkey on several occasions, as its CP sequences are among the terminal branches of three of the most sampled CP phylogroups. The complete PNRSV genome consists of three segments (RNA1, RNA2, and RNA3), with the larger two encoding replicases and the smallest encoding the movement protein and the CP. One quarter of the RNA1 and RNA2 genes were recombinants. The phylogenies of the CP and MP genes (i.e., different regions of RNA3) were closely correlated but did not correlate with those of RNA1 and RNA2, indicating that some of the isolates were reassortants. However, the non-reassortant ancestor could not be identified, probably because none of the complete genome sequences were from isolates from the basal CP phylogroups. Our results emphasize the importance of strict quarantine, both international and local, for the world's stone fruit crops.



Similar content being viewed by others
Availability of data and material
The data supporting the findings of this study are included in this published article (and/or) its supplementary information. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Verma N, Hallan V, Ram R, Zaidi AA (2002) Detection of Prunus necrotic ringspot virus in begonia by RT-PCR. Plant Pathol 51(6):800. https://doi.org/10.1046/j.1365-3059.2002.00770.x
Fiore N, Fajardo TVM, Prodan S, Herranz MC, Aparicio F, Montealegre J et al (2008) Genetic diversity of the movement and coat protein genes of South American isolates of Prunus necrotic ringspot virus. Arch Virol 153:909–919. https://doi.org/10.1007/s00705-008-0066-1
Cui HG, Liu HZ, Chen J, Zhou JF, Qu LN, Suc JM et al (2015) Genetic diversity of Prunus necrotic ringspot virus infecting stone fruit trees grown at seven regions in China and differentiation of three phylogroups by multiplex RT-PCR. Crop Prot 74:30–36. https://doi.org/10.1016/j.cropro.2015.04.001
Çelik A, Ertunç F (2019) First report of prunus necrotic ringspot virus infecting apple in Turkey. J Plant Pathol 101(4):1227. https://doi.org/10.1007/s42161-019-00286-7
Karanfil A (2021) Prevalence and molecular characterization of Turkish isolates of the rose viruses. Crop Prot 143:105565. https://doi.org/10.1016/j.cropro.2021.105565
Fulton RW (1985) Prunus necrotic ringspot ilarvirus. CMI/AAB Descriptions of Plant Viruses. Association of Applied Biologists, Wellesbourne, UK.
Amari K, Burgos L, Pallás V, Sánchez-Pina MA (2009) Vertical transmission of Prunus necrotic ringspot virus: hitch-hiking from gametes to seedling. J Gen Virol 90(7):1767–1774. https://doi.org/10.1099/vir.0.009647-0
Jones RAC (2018) Plant and insect viruses in managed and natural environments: novel and neglected transmission pathways. Adv Virus Res 101:149–187. https://doi.org/10.1016/bs.aivir.2018.02.006
Aparicio F, Myrta A, Di Terlizzi B, Pallás V (1999) Molecular variability among isolates of Prunus necrotic ringspot virus from different Prunus spp. Phytopathology 89(11):991–999. https://doi.org/10.1094/phyto.1999.89.11.991
Kamenova I, Borisova A (2021) Molecular variability of the coat protein gene of prunus necrotic ringspot virus on sweet and sour cherry in Bulgaria. J Plant Pathol 103(1):97–104. https://doi.org/10.1007/s42161-020-00659-3
Sokhandan-Bashir N, Kashiha M, Koolivand D, Eini O (2017) Detection and phylogenetic analysis of Prunus necrotic ringspot virus isolates from stone fruits in Iran. J Plant Pathol 99(3):1–14. https://doi.org/10.4454/jpp.v99i3.3986
Kulshrestha S, Hallan V, Sharma A, Seth CA, Chauhan A, Zaidi AA (2013) Molecular characterization and intermolecular interaction of coat protein of Prunus necrotic ringspot virus: implications for virus assembly. Indian J Virol 24(2):235–241. https://doi.org/10.1007/s13337-013-0140-5
Fajardo TVM, Nascimento MB, Eiras M, Nickel O, Pio-Ribeiro G (2015) Molecular characterization of Prunus necrotic ringspot virus isolated from rose in Brazil. Cienc Rural 45(12):2197–2200. https://doi.org/10.1590/0103-8478cr20141810
Boulila M, Tiba SB, Jilani S (2013) Molecular adaptation within the coat protein-encoding gene of Tunisian almond isolates of Prunus necrotic ringspot virus. J Genet 92:11–24. https://doi.org/10.1007/s12041-013-0211-9
Glasa M, Betinová E, Kúdela O, Šubr Z (2002) Biological and molecular characterisation of Prunus necrotic ringspot virus isolates and possible approaches to their phylogenetic typing. Ann Appl Biol 140:279–283. https://doi.org/10.1111/j.1744-7348.2002.tb00182.x
Song S, Sun P, Chen Y, Ma Q, Wang X, Zhao M, Li Z (2019) Complete genome sequences of five prunus necrotic ringspot virus isolates from Inner Mongolia of China and comparison to other PNRSV isolates around the world. J Plant Pathol 101(4):1047–1054. https://doi.org/10.1007/s42161-019-00335-1
Xing F, Gao D, Liu H, Wang H, Habili N, Li S (2020) Molecular characterization and pathogenicity analysis of prunus necrotic ringspot virus isolates from China rose (Rosa chinensis Jacq.). Arch Virol. 165(11):2479–2486. https://doi.org/10.1007/s00705-020-04739-8
Ulubas C, Ertunc F (2004) RT-PCR detection and molecular characterization of Prunus necrotic ringspot virus isolates occurring in Turkey. J Phytopathol 152(8–9):498–502. https://doi.org/10.1111/J.1439-0434.2004.00886.X
Çevik B, Yardimci N, Çulal-Kılıç H (2011) Detection of viruses infecting stone fruits in Western Mediterranean Region of Turkey. Plant Pathol J 27(1):44–52. https://doi.org/10.5423/PPJ.2011.27.1.044
Spiegel S, Tam Y, Maslenin L, Kolber M, Nemeth M, Rosner A (1999) Typing Prunus necrotic ringspot virus isolates by serology and restriction endonuclease analysis of PCR products. Ann Appl Biol 135(1):395–400. https://doi.org/10.1111/j.1744-7348.1999.tb00866.x
Hall TA (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–15492021
Abascal F, Zardoya R, Telford MJ (2010) TranslatorX: multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 38(Web Server issue):W7–W13. https://doi.org/10.1093/nar/gkq291
Katoh K, Standley DM (2013) MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol 30(4):772–780. https://doi.org/10.1093/molbev/mst010
Maynard-Smith JM (1992) Analyzing the mosaic structure of genes. J Mol Evol 34:126–129. https://doi.org/10.1007/bf00182389
Holmes EC, Worobey M, Rambaut A (1999) Phylogenetic evidence for recombination in dengue virus. Mol Biol Evol 16:405–409. https://doi.org/10.1093/oxfordjournals.molbev.a026121
Gibbs MJ, Armstrong JS, Gibbs AJ (2000) Sister-Scanning: a Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics 16:573–582. https://doi.org/10.1093/bioinformatics/16.7.573
Martin DP, Rybicki E (2000) RDP: detection of recombination amongst aligned sequences. Bioinformatics 16:562–563. https://doi.org/10.1093/bioinformatics/16.6.562
McGuire G, Wright F (2000) TOPAL 2.0: Improved detection of mosaic sequences within multiple alignments. Bioinformatics 16:130–134. https://doi.org/10.1093/bioinformatics/16.2.130
Posada D, Crandall KA (2001) Evaluation of methods for detecting recombination from DNA sequences: computer simulations. PNAS 98:13757–13762. https://doi.org/10.1073/pnas.241370698
Martin DP, Posada D, Crandall KA, Williamson C (2005) A modified bootscan algorithm for automated identification of recombinant sequences and recombination breakpoints. AIDS Res. Human Retroviruses 21:98–102. https://doi.org/10.1089/aid.2005.21.98
Boni MF, Posada D, Feldman MW (2007) An exact nonparametric method for inferring mosaic structure in sequence triplets. Genetics 176:1035–1047. https://doi.org/10.1534/genetics.106.068874
Lemey P, Lott M, Martin DP, Moulton V (2009) Identifying recombinants in human and primate immunodeficiency virus sequence alignments using quartet scanning. BMC Bioinformatics 10:126. https://doi.org/10.1186/1471-2105-10-126
Martin DP, Murrell B, Golden M, Khoosal A, Muhire B (2015) RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 1:1–5. https://doi.org/10.1093/ve/vev003
Tavaré S (1986) Some probabilistic and statistical problems in the analysis of DNA sequences. Lect. Math. Life Sci. 17:57–86
Fourment M, Gibbs MJ (2006) PATRISTIC: a program for calculating patristic distances and graphically comparing the components of genetic change. BMC Evol Biol 6:1. https://doi.org/10.1186/1471-2148-6-1
Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23(10):403–405. https://doi.org/10.1016/s0968-0004(98)01285-7
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biol 52:696–704. https://doi.org/10.1080/10635150390235520
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/bf01731581
Shimodaira H, Hasegawa M (1999) Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol 16:114–116. https://doi.org/10.1093/oxfordjournals.molbev.a026201
Muhire BM, Varsani A, Martin DP (2014) SDT: a virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 9(9):e108277. https://doi.org/10.1371/journal.pone.0108277
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sanchez-Gracia A (2017) DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol 34:3299–3302. https://doi.org/10.1093/molbev/msx248
Fu YX, Li WH (1993) Statistical tests of neutrality of mutations. Genetics 133(3):693–709. https://doi.org/10.1093/genetics/133.3.693
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123(3):585–595
Hudson RR (2000) A new statistic for detecting genetic differentiation. Genetics 155:2011–2014
Santosa AI, Ertunc F (2021) Phylogenetic and diversity analyses of Garlic common latent virus based on the TGB and CP gene sequence. Plant Prot Sci. 57(3):179–187. https://doi.org/10.17221/149/2020-PPS
Simmonds P, Aiewsakun P (2018) Virus classification - where do you draw the line? Arch Virol 163:2037–2046. https://doi.org/10.1007/s00705-018-3938-z
Wright S (1943) Isolation by distance. Genetics 28(2):114–138
Hartl DL, Clark AG (1997) Principles of Population Genetics. Sinauer Associates, Sunderland
Hammond RW, Crosslin JM (1998) Virulence and molecular polymorphism of Prunus necrotic ringspot virus isolates. J Gen Virol 79(7):1815–1823. https://doi.org/10.1099/0022-1317-79-7-1815
Hajizadeh M, Gibbs AJ, Amirnia F, Glasa M (2019) The global phylogeny of Plum pox virus is emerging. J Gen Virol 100:1457–1468. https://doi.org/10.1099/jgv.0.001308
Acknowledgements
A.Ç. provided personal funding to conduct field surveys and laboratory work in this study. This research is a part of the Doctoral dissertation of A.Ç. which was carried out in the Institute of Science at Ankara University, Turkey.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. A.Ç. performed field surveys, serological and molecular detection tests, and wrote the manuscript. A.Ç., A.I.S. and A.J.G. prepared sequence alignments, did phylogenetic and evolutionary analysis, and wrote and edited the manuscript. F.E. supervised the laboratory work and other analyses and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Elvira Fiallo-Olivé.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Çelik, A., Santosa, A.I., Gibbs, A.J. et al. Prunus necrotic ringspot virus in Turkey: an immigrant population. Arch Virol 167, 553–562 (2022). https://doi.org/10.1007/s00705-022-05374-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-022-05374-1