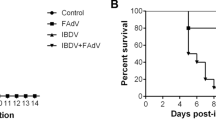
Abstract
Hepatitis-hydropericardium syndrome (HHS), caused by fowl adenovirus serotype 4 (FAdV-4), has spread on chicken farms worldwide, causing huge economic losses. Currently, the exact mechanism of pathogenesis of FAdV-4 remains unknown. Despite the severe inflammatory damage observed in chickens infected with pathogenic FAdV-4, few studies have focused on the host immune system-virus interactions and cytokine secretion. Host immunity acts as one of the most robust defense mechanisms against infection by pathogens, and cytokines are important in their elimination. However, excessive inflammatory cytokine secretion could contribute to the pathogenesis of FAdV-4. Understanding of the roles of cytokines produced during FAdV-4 infection is important for the study of pathogenicity and for developing strategies to control FAdV-4. Several previous studies have addressed the immune responses to FAdV-4 infection, but there has not been a systematic review of this work. The present review provides a detailed summary of the current findings on cytokine production induced by FAdV-4 infection to accelerate our understanding of FAdV-4 pathogenesis.
Similar content being viewed by others
References
Hess M (2000) Detection and differentiation of avian adenoviruses: a review. Avian Pathol 29:195–206. https://doi.org/10.1080/03079450050045440
Schachner A, Matos M, Grafl B, Hess M (2018) Fowl adenovirus-induced diseases and strategies for their control—a review on the current global situation. Avian Pathol 47:111–126. https://doi.org/10.1080/03079457.2017.1385724
Shah MS, Ashraf A, Khan MI et al (2017) Fowl adenovirus: history, emergence, biology and development of a vaccine against hydropericardium syndrome. Adv Virol 162:1833–1843. https://doi.org/10.1007/s00705-017-3313-5
Niu YJ, Sun W, Zhang GH et al (2016) Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. J Gen Virol 97:2684–2690. https://doi.org/10.1099/jgv.0.000567
Wang Z, Zhao J (2019) Pathogenesis of hypervirulent fowl adenovirus serotype 4: the contributions of viral and host factors. Viruses. https://doi.org/10.3390/v11080741
Schonewille E, Singh A, Göbel TW et al (2008) Fowl adenovirus (FAdV) serotype 4 causes depletion of B and T cells in lymphoid organs in specific pathogen-free chickens following experimental infection. Vet Immunol Immunopathol 121:130–139. https://doi.org/10.1016/j.vetimm.2007.09.017
Niu Y, Sun Q, Zhang G et al (2018) Epidemiological investigation of outbreaks of fowl adenovirus infections in commercial chickens in China. Transbound Emerg Dis 65:e121–e126. https://doi.org/10.1111/tbed.12691
Ojkic D, Martin E, Swinton J et al (2008) Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathol 37:95–100. https://doi.org/10.1080/03079450701805324
Yan T, Zhu S, Wang H et al (2020) Synergistic pathogenicity in sequential coinfection with fowl adenovirus type 4 and avian orthoreovirus. Vet Microbiol 251:108880. https://doi.org/10.1016/j.vetmic.2020.108880
Jiang Z, Liu M, Wang C et al (2019) Characterization of fowl adenovirus serotype 4 circulating in chickens in China. Vet Microbiol 238:108427. https://doi.org/10.1016/j.vetmic.2019.108427
Su Q, Li Y, Meng F et al (2018) Newcastle disease virus-attenuated vaccine co-contaminated with fowl adenovirus and chicken infectious anemia virus results in inclusion body hepatitis-hydropericardium syndrome in poultry. Vet Microbiol. https://doi.org/10.1016/j.vetmic.2018.03.019
Toro H, González O, Escobar C et al (2001) Vertical induction of the inclusion body hepatitis/hydropericardium syndrome with fowl adenovirus and chicken anemia virus. Avian Dis 45:215–222. https://doi.org/10.2307/1593031
Su Q, Meng F, Li Y et al (2019) Chicken infectious anemia virus helps fowl adenovirus break the protection of maternal antibody and cause inclusion body hepatitis-hydropericardium syndrome in layers after using co-contaminated Newcastle disease virus-attenuated vaccine. Poult Sci. https://doi.org/10.3382/ps/pey153
Neerukonda SN, Katneni U (2020) Avian pattern recognition receptor sensing and signaling. Vet Sci 7:1–41. https://doi.org/10.3390/vetsci7010014
Brownlie R, Allan B (2011) Avian toll-like receptors. Cell Tissue Res 343:121–130. https://doi.org/10.1007/s00441-010-1026-0
Chen S, Cheng A, Wang M (2013) Innate sensing of viruses by pattern recognition receptors in birds. Vet Res 44:1–12. https://doi.org/10.1186/1297-9716-44-82
Nawab A, An L, Wu J et al (2019) Chicken toll-like receptors and their significance in immune response and disease resistance. Int Rev Immunol 38:284–306. https://doi.org/10.1080/08830185.2019.1659258
Higgs R, Cormican P, Cahalane S et al (2006) Induction of a novel chicken Toll-like receptor following Salmonella enterica serovar Typhimurium infection. Infect Immun 74:1692–1698. https://doi.org/10.1128/IAI.74.3.1692-1698.2006
Brownlie R, Zhu J, Allan B et al (2009) Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol Immunol 46:3163–3170. https://doi.org/10.1016/j.molimm.2009.06.002
Zhao W, Li X, Li H et al (2020) Fowl adenoviruse-4 infection induces strong innate immune responses in chicken. Comp Immunol Microbiol Infect Dis 68:101404. https://doi.org/10.1016/j.cimid.2019.101404
Meng K, Yuan X, Yu J, et al (2019) Identification, Pathogenicity of Novel Fowl Adenovirus Serotype 4 SDJN0105 in Shandong, China and Immunoprotective Evaluation of the Newly Developed Inactivated Oil-emulsion FAdV-4 Vaccine. Viruses 11
Li R, Li G, Lin J et al (2018) Fowl adenovirus serotype 4 SD0828 infections causes high mortality rate and cytokine levels in specific pathogen-free chickens compared to ducks. Front Immunol. https://doi.org/10.3389/fimmu.2018.00049
Srinivasula SM, Poyet JL, Razmara M et al (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem 277:21119–21122. https://doi.org/10.1074/jbc.C200179200
Wei S, Ma W, Zhang B, Li W (2021) NLRP3 inflammasome: a promising therapeutic target for drug-induced toxicity. Front Cell Dev Biol 9:1–20. https://doi.org/10.3389/fcell.2021.634607
Li M, Raheem MA, Han C et al (2020) The fowl adenovirus serotype 4 ( FAdV-4) induce cellular pathway in chickens to produce interferon and antigen-presented molecules ( MHCI / II ). Poult Sci 100:101406. https://doi.org/10.1016/j.psj.2021.101406
Yazdi AS, Ghoreschi K (2016) The interleukin-1 family. Adv Exp Med Biol 941:21–29. https://doi.org/10.1007/978-94-024-0921-5_2
Niu Y, Sun Q, Zhang G et al (2018) Fowl adenovirus serotype 4-induced apoptosis, autophagy, and a severe inflammatory response in liver. Vet Microbiol 223:34–41. https://doi.org/10.1016/j.vetmic.2018.07.014
Niu Y, Sun Q, Liu X, Liu S (2019) Mechanism of fowl adenovirus serotype 4-induced heart damage and formation of pericardial effusion. Poult Sci 98:1134–1145. https://doi.org/10.3382/ps/pey485
Grgić H, Poljak Z, Sharif S, Nagy É (2013) Pathogenicity and cytokine gene expression pattern of a serotype 4 fowl adenovirus isolate. PLoS ONE 8:1–10. https://doi.org/10.1371/journal.pone.0077601
Palomo J, Dietrich D, Martin P et al (2015) The interleukin (IL)-1 cytokine family—balance between agonists and antagonists in inflammatory diseases. Cytokine 76:25–37. https://doi.org/10.1016/j.cyto.2015.06.017
Rider P, Carmi Y, Guttman O et al (2011) IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol 187:4835–4843. https://doi.org/10.4049/jimmunol.1102048
Bent R, Moll L, Grabbe S, Bros M (2018) Interleukin-1 beta—a friend or foe in malignancies? Int J Mol Sci
Miura K, Kodama Y, Inokuchi S et al (2010) Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology 139:323–334. https://doi.org/10.1053/j.gastro.2010.03.052.MIURA
Dinarello CA, van der Meer JWM (2011) Treating inflammation by blocking interleukin-1 in humans. Semin Immunol 4:469–484. https://doi.org/10.1016/j.smim.2013.10.008.Treating
Shumer DE, Spack NPNJN (2017) Macrophage autophagy limits acute toxic liver injury in mice through down regulation of interleukin-1β. Physiol Behav 176:139–148. https://doi.org/10.1016/j.physbeh.2017.03.040
Amarasinghe A, Abdul-Cader MS, Almatrouk Z et al (2018) Induction of innate host responses characterized by production of interleukin (IL)-1β and recruitment of macrophages to the respiratory tract of chickens following infection with infectious bronchitis virus (IBV). Vet Microbiol 215:1–10. https://doi.org/10.1016/j.vetmic.2018.01.001
Gao P, Chen L, Fan L et al (2020) Newcastle disease virus RNA-induced IL-1β expression via the NLRP3/caspase-1 inflammasome. Vet Res 51:1–14. https://doi.org/10.1186/s13567-020-00774-0
Xu J, Deng TL, Li L et al (2005) Nitric oxide inducing function and intracellular movement of chicken interleukin-18 in cultured cells. Acta Biochim Biophys Sin 37:688–693. https://doi.org/10.1111/j.1745-7270.2005.00098.x
Esmailbeig M, Ghaderi A (2017) Interleukin-18: a regulator of cancer and autoimmune diseases. Eur Cytokine Netw 28:127–140. https://doi.org/10.1684/ecn.2018.0401
Hung LH, Li HP, Lien YY et al (2010) Adjuvant effects of chicken interleukin-18 in avian Newcastle disease vaccine. Vaccine 28:1148–1155. https://doi.org/10.1016/j.vaccine.2009.11.042
Rahman MM, Uyangaa E, Eo SK (2013) Modulation of humoral and cell-mediated immunity against avian influenza and newcastle disease vaccines by oral administration of Salmonella enterica serovar typhimurium expressing chicken interleukin-18. Immune Network 13:34. https://doi.org/10.4110/in.2013.13.1.34
Rahman MM, Uyangaa E, Han YW et al (2012) Enhancement of Th1-biased protective immunity against avian influenza H9N2 virus via oral co-administration of attenuated Salmonella enterica serovar Typhimurium expressing chicken interferon-α and interleukin-18 along with an inactivated vaccine. BMC Vet Res 8:1–11. https://doi.org/10.1186/1746-6148-8-105
Li K, Gao H, Gao L et al (2013) Adjuvant effects of interleukin-18 in DNA vaccination against infectious bursal disease virus in chickens. Vaccine 31:1799–1805. https://doi.org/10.1016/j.vaccine.2013.01.056
Chen HY, Zhao L, Wei ZY et al (2010) Enhancement of the immunogenicity of an infectious laryngotracheitis virus DNA vaccine by a bicistronic plasmid encoding glycoprotein B and interleukin-18. Antiviral Res 87:235–241. https://doi.org/10.1016/j.antiviral.2010.05.009
Song B, Li X, Ma J et al (2018) Prokaryotic expression and Anti-IBDV activity of chicken interleukin-18 and interferon-γ. Cytogenet Genome Res 153:36–45. https://doi.org/10.1159/000481522
Niu Y, Sun Q, Shi Y et al (2019) Immunosuppressive potential of fowl adenovirus serotype 4. Poult Sci 98:3514–3522. https://doi.org/10.3382/ps/pez179
Copaescu A, Smibert O, Gibson A et al (2020) The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol 146:519–534
Schindler C, Darnell JE (1995) Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 64:621–651. https://doi.org/10.1146/annurev.bi.64.070195.003201
Gao Y, Zhang Y, Yao Y et al (2016) Avian leukosis virus subgroup J induces VEGF expression via NF-κB/PI3K-dependent IL-6 production. Oncotarget 7:80275–80287. https://doi.org/10.18632/oncotarget.13282
Sun JH, Yan YX, Jiang J, Lu P (2005) DNA immunization against very virulent infectious bursal disease virus with VP2-4-3 gene and chicken IL-6 gene. J Vet Med Ser B Infect Dis Vet Public Health 52:1–7. https://doi.org/10.1111/j.1439-0450.2004.00813.x
Wu YF, Shien JH, Yin HH et al (2008) Structural and functional homology among chicken, duck, goose, turkey and pigeon interleukin-8 proteins. Vet Immunol Immunopathol 125:205–215. https://doi.org/10.1016/j.vetimm.2008.03.001
Carla Piazzon M, Lutfall G, Forlenzaa M (2016) IL10, a tale of an evolutionarily conserved cytokine across vertebrates. Crit Rev Immunol 36:99–129. https://doi.org/10.1615/CritRevImmunol.2016017480
Wajant H, Pfizenmaier K, Scheurich P (2003) Tumor necrosis factor signaling. Cell Death Differ 10:45–65. https://doi.org/10.1038/sj.cdd.4401189
Jeurissen SHM, Boonstra-Blom AG, Al-Garib SO et al (2000) Defence mechanisms against viral infection in poultry: a review. Vet Q 22:204–208. https://doi.org/10.1080/01652176.2000.9695059
Cui J, Xu Y, Zhou Z et al (2020) Pathogenicity and molecular typing of fowl adenovirus-associated with hepatitis/hydropericardium syndrome in Central China (2015–2018). Front Vet Sci 7:1–10. https://doi.org/10.3389/fvets.2020.00190
Wu N, Yang B, Wen B et al (2020) Pathogenicity and immune responses in specific-pathogen-free chickens during fowl adenovirus serotype 4 infection. Avian Dis 64:315–323. https://doi.org/10.1637/aviandiseases-D-20-00004
Hilton LS, Bean AGD, Kimpton WG, Lowenthal JW (2002) Interleukin-2 directly induces activation and proliferation of chicken T cells in vivo. J Interferon Cytokine Res 22:755–763. https://doi.org/10.1089/107999002320271341
Susta L, Diel DG, Courtney S et al (2015) Expression of chicken interleukin-2 by a highly virulent strain of Newcastle disease virus leads to decreased systemic viral load but does not significantly affect mortality in chickens. Virol J 12:1–17. https://doi.org/10.1186/s12985-015-0353-x
Huo S, Zhang J, Fan J et al (2019) Co-expression of chicken il-2 and il-7 enhances the immunogenicity and protective efficacy of a vp2-expressing dna vaccine against ibdv in chickens. Viruses. https://doi.org/10.3390/v11050476
Chaudhari AA, Kim WH, Lillehoj HS (2018) Interleukin-4 (IL-4) may regulate alternative activation of macrophage-like cells in chickens: a sequential study using novel and specific neutralizing monoclonal antibodies against chicken IL-4. Vet Immunol Immunopathol 205:72–82. https://doi.org/10.1016/j.vetimm.2018.10.011
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35. https://doi.org/10.1038/nri978
Sawant PM, Verma PC, Subudhi PK et al (2011) Immunomodulation of bivalent Newcastle disease DNA vaccine induced immune response by co-delivery of chicken IFN-γ and IL-4 genes. Vet Immunol Immunopathol 144:36–44. https://doi.org/10.1016/j.vetimm.2011.07.006
Jiang H, Yang H, Kapczynski DR (2011) Chicken interferon alpha pretreatment reduces virus replication of pandemic H1N1 and H5N9 avian influenza viruses in lung cell cultures from different avian species. Virol J 8:1–12. https://doi.org/10.1186/1743-422X-8-447
Swanson KV, Deng M, Ting JP-Y (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19:477–489. https://doi.org/10.1038/s41577-019-0165-0.The
Nilea SH, Nilea A, Qiua J et al (2020) COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 54:66–70
Chen L, Deng H, Cui H et al (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9:7204–7218
Asif M, Jenkins KA, Hilton LS et al (2004) Cytokines as adjuvants for avian vaccines. Immunol Cell Biol 82:638–643. https://doi.org/10.1111/j.1440-1711.2004.01295.x
Yu G, Wang Y, Zhang M et al (2018) Pathogenic, phylogenetic, and serological analysis of Group i fowl adenovirus serotype 4 SDSX isolated from Shandong, China. Front Microbiol 9:1–12. https://doi.org/10.3389/fmicb.2018.02772
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 31772771).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Eric J Kremer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, B., Guo, H. & Zhao, J. The pros and cons of cytokines for fowl adenovirus serotype 4 infection. Arch Virol 167, 281–292 (2022). https://doi.org/10.1007/s00705-021-05318-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-021-05318-1