Abstract
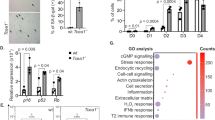
Recently, it was reported that the forkhead box O (FoxO) transcription factor promotes human cytomegalovirus (HCMV) replication via direct binding to the promoters of the major immediate-early (MIE) genes, but how the FoxO factor impacts HCMV replication remains unknown. Here, it is reported that FoxO1 expression is strongly induced by HCMV infection in cells of fibroblast origin. Suppression of the FoxO1 gene by specific RNA interference significantly inhibited HCMV growth and replication, but viral DNA synthesis was not affected considerably. Interestingly, depletion or overexpression of FoxO1 had a significant effect on the expression of viral early/late transcripts. FoxO1 was found to colocalize with the pUL44 protein subunit of viral replication compartments without direct association with DNA. This study highlights how FoxO enhances HCMV gene transcription and viral replication to promote infection.




Similar content being viewed by others
Abbreviations
- HCMV:
-
Human cytomegalovirus
- IE:
-
Immediate early gene
- E:
-
Early gene
- EL:
-
Early-late gene
- L:
-
Late gene
- FoxO:
-
Forkhead box O transcription factor
- BAC:
-
Bacterial artificial chromosome
- vRC:
-
Viral replication compartment
- EBV:
-
Epstein-Barr virus
- HCV:
-
Hepatitis C virus
- FoxO1–ADA:
-
FoxO1 containing T24A/S253D/S316A mutations
- GFP:
-
Green fluorescent protein
- ChIP:
-
Chromatin immunoprecipitation
- IGFBP1:
-
Insulin-like growth factor binding protein 1
- HEK293T:
-
Human embryonic kidney 293 T cell
- MRC5:
-
Medical Research Council cell strain 5 (human embryonic lung fibroblasts
References
Mocarski ES Jr, Shenk T, Pass RF (2007) Cytomegaloviruses. Fields Virol II:2701–2772
Crumpacker CS (2015) Cytomegalovirus (CMV). In: Bennett J, Dolin R, Blaser M (eds) Bennett’s principles and practice of infectious diseases, 8th edn. Saunders, New York, pp 1738-1753.e4
Fehr AR, Yu D (2013) Control the host cell cycle: viral regulation of the anaphase-promoting complex. J Virol 87:8818–8825
Sanchez V, Spector DH (2008) Subversion of cell cycle regulatory pathways. Curr Top Microbiol Immunol 325:243–262
Yu Y, Pierciey FJ Jr, Maguire TG, Alwine JC (2013) PKR-like endoplasmic reticulum kinase is necessary for lipogenic activation during HCMV infection. PLoS Pathog 9:e1003266
Yu Y, Maguire TG, Alwine JC (2014) ChREBP, a glucose-responsive transcriptional factor, enhances glucose metabolism to support biosynthesis in human cytomegalovirus-infected cells. Proc Natl Acad Sci USA 111:1951–1956
Yu Y, Clippinger AJ, Alwine JC (2011) Viral effects on metabolism: changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol 19:360–367
Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD (2008) Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol 26:1179–1186
Munger J, Bajad SU, Coller HA, Shenk T, Rabinowitz JD (2006) Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog 2:e132
DeVito SR, Ortiz-Riano E, Martinez-Sobrido L, Munger J (2014) Cytomegalovirus-mediated activation of pyrimidine biosynthesis drives UDP-sugar synthesis to support viral protein glycosylation. Proc Natl Acad Sci USA 111:18019–18024
Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC (2008) The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol 6:266–275
Clippinger AJ, Alwine JC (2012) Dynein mediates the localization and activation of mTOR in normal and human cytomegalovirus-infected cells. Genes Dev 26:2015–2026
Clippinger AJ, Maguire TG, Alwine JC (2011) Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation. J Virol 85:9369–9376
Clippinger AJ, Maguire TG, Alwine JC (2011) The changing role of mTOR kinase in the maintenance of protein synthesis during human cytomegalovirus infection. J Virol 85:3930–3939
Tilton C, Clippinger AJ, Maguire T, Alwine JC (2011) Human cytomegalovirus induces multiple means to combat reactive oxygen species. J Virol 85:12585–12593
Alwine JC (2008) Modulation of host cell stress responses by human cytomegalovirus. Curr Top Microbiol Immunol 325:263–279
Qian Z, Xuan B, Gualberto N, Yu D (2011) The human cytomegalovirus protein pUL38 suppresses endoplasmic reticulum stress-mediated cell death independently of its ability to induce mTORC1 activation. J Virol 85:9103–9113
Terhune S, Torigoi E, Moorman N, Silva M, Qian Z, Shenk T, Yu D (2007) Human cytomegalovirus UL38 protein blocks apoptosis. J Virol 81:3109–3123
Eijkelenboom A, Burgering BM (2013) FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 14:83–97
Lam EW, Brosens JJ, Gomes AR, Koo CY (2013) Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer 13:482–495
Webb AE, Kundaje A, Brunet A (2016) Characterization of the direct targets of FOXO transcription factors throughout evolution. Aging Cell 15(4):673–685
Tzivion G, Dobson M, Ramakrishnan G (2011) FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta 1813:1938–1945
Hay N (2011) Interplay between FOXO, TOR, and Akt. Biochem Biophys Acta 1813(11):1965–1970
Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23:4802–4812
Yuan Z, Lehtinen MK, Merlo P, Villén J, Gygi S, Bonni A (2009) Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem 284(17):11285–11292
Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EBE, DiBacco S, de la Iglesia N, Gygi SP, Blackwell TK, Bonni A (2006) A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125:987–1001
Calnan DR, Brunet A (2008) The FoxO code. Oncogene 27:2276–2288
Munoz-Fontela C, Marcos-Villar L, Gallego P, Arroyo J, Da Costa M, Pomeranz KM, Lam EW, Rivas C (2007) Latent protein LANA2 from Kaposi’s sarcoma-associated herpesvirus interacts with 14-3-3 proteins and inhibits FOXO3a transcription factor. J Virol 81:1511–1516
Chen YR, Liu MT, Chang YT, Wu CC, Hu CY, Chen JY (2008) Epstein-Barr virus latent membrane protein 1 represses DNA repair through the PI3K/Akt/FOXO3a pathway in human epithelial cells. J Virol 82:8124–8137
Deng L, Shoji I, Ogawa W, Kaneda S, Soga T, Jiang DP, Ide YH, Hotta H (2011) Hepatitis C virus infection promotes hepatic gluconeogenesis through an NS5A-mediated, FoxO1-dependent pathway. J Virol 85:8556–8568
Oteiza A, Mechti N (2011) The human T-cell leukemia virus type 1 oncoprotein tax controls forkhead box O4 activity through degradation by the proteasome. J Virol 85:6480–6491
van Grevenynghe J, Procopio FA, He Z, Chomont N, Riou C, Zhang Y et al (2008) Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat Med 14:266–274
Cui M, Huang Y, Zhao Y, Zheng J (2009) New insights for FOXO and cell-fate decision in HIV infection and HIV associated neurocognitive disorder. Adv Exp Med Biol 665:143–159
Kino T, De Martino MU, Charmandari E, Ichijo T, Outas T, Chrousos GP (2005) HIV-1 accessory protein Vpr inhibits the effect of insulin on the FoxO subfamily of forkhead transcription factors by interfering with their binding to 14-3-3 proteins: potential clinical implications regarding the insulin resistance of HIV-1-infected patients. Diabetes 54:23–31
Wilk A, Urbanska K, Yang S, Wang JY, Amini S, Del Valle L, Peruzzi F, Meggs L, Reiss K (2011) Insulin-like growth factor-I-forkhead box O transcription factor 3a counteracts high glucose/tumor necrosis factor-alpha-mediated neuronal damage: implications for human immunodeficiency virus encephalitis. J Neurosci Res 89:183–198
Zhang S, Liu L, Wang R, Tuo H, Guo Y, Yi L, Wang D, Wang J (2013) MicroRNA-217 promotes the angiogenesis of human cytomegalovirus-infected endothelial cells through downregulation of SIRT1 and FOXO3A. PLoS ONE 8:e83620
Adamson CS, Nevels MM (2020) Bright and early: inhibiting human cytomegalovirus by targeting major immediate-early gene expression or protein function. Viruses 12(1):110. https://doi.org/10.3390/v12010110
Hale AE, Collins-McMillen D, Lenarcic EM et al (2020) FOXO transcription factors activate alternative major immediate-early promoters to induce human cytomegalovirus reactivation. PNAS 117(31):18764–18770
Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC (2010) Transcription factors FoxO3a and FoxO1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med 207:1381–1391
Qian Z, Leung-Pineda V, Xuan B, Piwnica-Worms H, Yu D (2010) Human cytomegalovirus protein pUL117 tar (Baar MP, 2017) gets the mini-chromosome maintenance complex and suppresses cellular DNA synthesis. PLoS Pathog 6:e1000814
Everett RD, Boutell C, McNair C, Grant L, Orr A (2010) Comparison of the biological and biochemical activities of several members of the alphaherpesvirus ICP0 family of proteins. J Virol 84:3476–3487
Boussif O et al (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 92:7297–7301
Fehr AR, Gualberto NC, Savaryn JP, Terhune SS, Yu D (2012) Proteasome-dependent disruption of the E3 ubiquitin ligase anaphase-promoting complex by HCMV protein pUL21a. PLoS Pathog 8:e1002789
Nakae J, Kitamura T, Silver DL, Accili D (2001) The forkhead transcription factor FoxO1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 108:1359–1367
Yu D, Smith GA, Enquist LW, Shenk T (2002) Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J Virol 76:2316–2328
Xuan B, Qian Z, Torigoi E, Yu D (2009) Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J Virol 83:3463–3474
Nelson J, Denisenko O, Bomsztyk K (2006) Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protoc 1:179–185
Yalley A, Schill D, Hatta M, Johnson N, Cirillo LA (2016) Loss of interdependent binding by the FoxO1 and FoxA1/A2 forkhead transcription factors culminates in perturbation of active chromatin marks and binding of transcriptional regulators at insulin-sensitive genes. J Biol Chem 291:8848–8861
Nakamura M, Takakura M, Fujii R, Maida Y, Bono Y, Mizumoto Y, Zhang X, Kiyono T, Kyo S (2013) The PRB-dependent FOXO1/IGFBP-1 axis is essential for progestin to inhibit endometrial epithelial growth. Cancer Lett 336(1):68–75
Hatta M, Cirillo LA (2007) Chromatin opening and stable perturbation of core histone:DNA contacts by FoxO1. J Biol Chem 282(49):35583–35593
Schill D, Nord J, Cirillo LA (2019) FoxO1 and FoxA1/2 form a complex on DNA and cooperate to open chromatin at insulin-regulated genes FoxO1 and FoxA1/2 form a complex on DNA and cooperate to open chromatin at insulin-regulated genes. Biochem Cell Biol 97(2):118–129
Perng YC, Qian Z, Fehr AR, Xuan B, Yu D (2011) The human cytomegalovirus gene UL79 is required for the accumulation of late viral transcripts. J Virol 85:4841–4852
Quinlan MP, Chen LB, Knipe DM (1984) The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36:857–868
Penfold ME, Mocarski ES (1997) Formation of cytomegalovirus DNA replication compartments defined by localization of viral proteins and DNA synthesis. Virology 239:46–61
Taylor TJ, Knipe DM (2004) Proteomics of herpes simplex virus replication compartments: association of cellular DNA replication, repair, recombination, and chromatin remodeling proteins with ICP8. J Virol 78:5856–5866
Qian Z, Xuan B, Hong TT, Yu D (2008) The full-length protein encoded by human cytomegalovirus gene UL117 is required for the proper maturation of viral replication compartments. J Virol 82:3452–3465
Isomura H, Stinski MF, Kudoh A et al (2007) The late promoter of the human cytomegalovirus viral DNA polymerase processivity factor has an impact on delayed early and late viral gene products but not on viral DNA synthesis. J Virol 81(12):6197–6206
Isomura H, Stinski MF, Kudoh A, Murata T, Nakayama S, Sato Y, Iwahori S, Tsurumi T (2008) Noncanonical TATA sequence in the UL44 late promoter of human cytomegalovirus is required for the accumulation of late viral transcripts. J Virol 82(4):1638–1646
Nevels M, Paulus C, Shenk T (2004) Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. PNAS 101(49):17234–17239
Acknowledgements
I first want to thank Shaowu Huang for his generous help in this report. Furthermore, I want to extend my thanks to Dr. Yongjun Yu (University of Pennsylvania) and Dr. Yi-Chieh Perng (Washington University in St. Louis) for reading the manuscript critically. I thank all of the Herpesvirus and Molecular Virology Research Unit members for helpful discussions, Dr. Thomas Shenk for antibodies, Dr. Jay Nelson for IE1/2 antibody, and Roger Everett (University of Glasgow) for the pLKO-based lentiviral expression system.
Funding
This work was supported by a CAS-TWAS Fellowship. The funders had no role in study design, data collection, analysis, or manuscript submission.
Author information
Authors and Affiliations
Contributions
SS performed experiments and wrote the manuscript, and he is the corresponding author.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Communicated by Graciela Andrei.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sleman, S. Virus-induced FoxO factor facilitates replication of human cytomegalovirus. Arch Virol 167, 109–121 (2022). https://doi.org/10.1007/s00705-021-05279-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-021-05279-5