Abstract
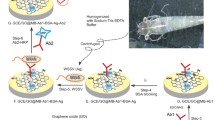
White spot syndrome virus (WSSV) is a significant threat to the aquaculture sector, causing mortality among crabs and shrimps. Currently available diagnostic tests for WSSV are not rapid or cost-effective, and a new detection method is therefore needed. This study demonstrates the development of a biosensor by functionalization of magnetosomes with VP28-specific antibodies to detect WSSV in seafood. The magnetosomes (1 and 2 mg/ml) were conjugated with VP28 antibody (0.025–10 ng/µl), as confirmed by spectroscopy. The magnetosome-antibody conjugate was used to detect the VP28 antigen. The binding of antigen to the magnetosome-antibody complex resulted in a change in absorbance. The magnetosome-antibody-antigen complex was then concentrated and brought near a screen-printed carbon electrode by applying an external magnetic field, and the antigen concentration was determined using impedance measurements. The VP28 antigen (0.025 ng/µl) bound more efficiently to the magnetosome-VP28 antibody complex (0.025 ng/µl) than to the VP28 antibody (0.1 ng/µl) alone. The same assay was repeated to detect the VP28 antigen (0.01 ng/µl) in WSSV-infected seafood samples using the magnetosome-VP28 antibody complex (0.025 ng/µl). The WSSV in the seafood sample was also drawn toward the electrode due to the action of magnetosomes controlled by the external magnetic field and detected using impedance measurement. The presence of WSSV in seafood samples was verified by Western blot and RT-PCR. Cross-reactivity assays with other viruses confirmed the specificity of the magnetosome-based biosensor. The results indicate that the use of the magnetosome-based biosensor is a sensitive, specific, and rapid way to detect WSSV in seafood samples.










Similar content being viewed by others
Availability of data and materials
The datasets in the current study are available from the corresponding author on request.
References
Guoxing Z, Yalin S, Kai Z, Zhi C (1997) Bacilliform virus infection in cultured Chinese shrimp, Penaeus orientalis, in China. J Mar Biotechnol 5:113–118
Sánchez-Paz A (2010) White spot syndrome virus: an overview on an emergent concern. Vet Res 41(6):43. https://doi.org/10.1051/vetres/2010015
Inouye K, Miwa S, Oseko N, Nakano H, Kimura T, Momoyama K, Hiraoka M (1994) Mass mortalities of cultured kuruma shrimp, Penaeus japonicus, in Japan in 1993: electron microscopic evidence of the causative virus. Fish Pathol 29:149–158. https://doi.org/10.3147/jsfp.29.149
Takahashi Y, Itami T, Kondo M, Maeda M, Fujii R, Tomonaga S, Supamattaya K, Boonyaratpalin S (1994) Electronmicroscopic evidence of bacilliform virus infection in Kuruma Shrimp (Penaeus japonicus). Fish Pathol 29(2):121–125. https://doi.org/10.3147/jsfp.29.121
Lo CF, Ho CH, Peng SE, Chen CH, Hsu HC, Chiu YL, Chang CF, Liu KF, Su MS, Wang CH, Kou GH (1996) White spot syndrome baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthroponds. Dis Aquat Org 27:215–225. https://doi.org/10.3354/dao027215
Karunasagar I, Otta SK, Karunasagar I (1997) Histopathological and bacteriological study of white spot syndrome of Penaeus monodon along the west coast on India. Aquaculture 153:9–13. https://doi.org/10.1016/S0044-8486(97)00011-2
Chou HY, Huang CY, Wang CH, Chiang HC, Lo CF (1995) Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis Aquat Org 23:165–173. https://doi.org/10.3354/dao023165
Li LJ, Yuan JF, Cai CA, Gu WG, Shi ZL (2006) Multiple envelope proteins are involved in white spot syndrome virus (WSSV) infection in crayfish. Arch Virol 151(7):1309–1317. https://doi.org/10.1007/s00705-005-0719-2
van Hulten MCW, Witteveldt J, Peters S, Kloosterboer N, Tarchini R, Fiers M (2001) The white spot syndrome virus DNA genome sequence. Virology 286:7–22. https://doi.org/10.1006/viro.2001.1002
Yi G, Wang Z, Qi Y, Yao L, Qian J, Hu L (2004) VP28 of shrimp white spot syndrome virus is involved in the attachment and penetration into shrimp cells. J Biochem Mol Biol 37:726–734. https://doi.org/10.5483/BMBRep.2004.37.6.726
Mathew S, Kumar KA, Anandan R, Nair PV, Devadasan K (2007) Changes in tissue defence system in white spot syndrome virus (WSSV) infected Penaeus monodon. Comp Biochem Physiol C Toxicol Pharmacol 145(3):315–320. https://doi.org/10.1016/j.cbpc.2007.01.001
Oakey J, Smith C, Underwood D, Afsharnasab M, Alday-Sanz V, Dhar A, Sivakumar S, Hameed AS, Beattie K, Crook A (2019) Global distribution of white spot syndrome virus genotypes determined using a novel genotyping assay. Arch Virol 164(8):2061–2082. https://doi.org/10.1007/s00705-019-04265-2
Shyam SS, Geetha R, Athira NR (2019) Economic recession and Indian seafood exports: reflections and paradigms. Indian J Econ Dev 71(12):1–9. http://ijed.informaticspublishing.com/index.php/ijed/article/view/147619/104552
Shen CF, Meghrous J, Kamen A (2002) Quantitation of baculovirus particles by flow cytometry. J Virol Methods 105(2):321–330. https://doi.org/10.1016/S0166-0934(02)00128-3
Wongprasert K, Sangsuriya P, Phongdara A, Senapin S (2007) Cloning and characterization of a caspase gene from black tiger shrimp (Penaeus monodon)-infected with white spot syndrome virus (WSSV). J Biotechnol 131(1):9–19. https://doi.org/10.1016/j.jbiotec.2007.05.032
Liu W, Wang TY, Tian SD, Yin CZ, Kwang J (2002) Detection of white spot syndrome virus (WSSV) of shrimp by means of monoclonal antibodies (MAbs) specific to an envelope protein (28 kDa). Dis Aquat Org 49:11–18. https://doi.org/10.3354/dao049011
Song KK, Li DF, Zhang MC, Yang HJ, Ruan LW, Xu X (2010) Cloning and characterization of three novel WSSV recognizing lectins from shrimp Marsupenaeus japonicus. Fish Shellfish Immunol 28(4):596–603. https://doi.org/10.1016/j.fsi.2009.12.015
Wang L, Zhi B, Wu W, Zhang X (2008) Requirement for shrimp caspase in apoptosis against virus infection. Dev Comp Immunol 32(6):706–715. https://doi.org/10.1016/j.dci.2007.10.010
Xie Z, Xie L, Pang Y, Lu Z, Xie Z, Sun J, Deng X, Liu J, Tang X, Khan M (2008) Development of a real-time multiplex PCR assay for detection of viral pathogens of penaeid shrimp. Arch Virol 153(12):2245–2251. https://doi.org/10.1007/s00705-008-0253-0
Sithigorngul W, Rukpratanporn S, Pecharaburanin N, Longyant S, Chaivisuthangkura P, Sithigorngul P (2006) A simple and rapid immunochromatographic test strip for detection of white spot syndrome virus (WSSV) of shrimp. Dis Aquat Org 72:101–106. https://doi.org/10.3354/dao072101
Fitzpatrick J, Fanning L, Hearty S, Leonard P, Manning BM, Quinn JQ, O’Kennedy R (2000) Applications and recent developments in the use of antibodies for analysis. Anal Lett 33(13):2563–2609. https://doi.org/10.1080/00032710008543210
Killard AJ, Smyth MR (2000) Separation-free electrochemical immunosensor strategies. Anal Lett 33(8):1451–1465. https://doi.org/10.1080/00032710008543135
Zhang S, Wang N, Yu H, Niu Y, Sun C (2005) Tailoring the surface potential of gold nanoparticles with self-assembled monolayers with mixed functional groups. Biochemistry 67:15–22. https://doi.org/10.1016/j.jcis.2009.08.014
Luo X, Morrin A, Killard Anthony J, Smyth Malcolm R (2006) Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 18(4):319–326. https://doi.org/10.1002/elan.200503415
Hussain MS, Hess LK, Gearhart MJ, Geiss TK, Schlager JJ (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro 19:975–983. https://doi.org/10.1016/j.tiv.2005.06.034
Salata OV (2004) Applications of nanoparticles in biology and medicine. J Nanobiotechnol 2(1):1. https://doi.org/10.1016/j.msec.2020.111071
Banerjee SK, Moskowitz BM (1985) Ferrimagnetic properties of magnetite. In: Kirschvink JL, Jones DS, MacFadden BM (eds) Magnetite biomineralization and magnetoreception in organisms. Plenum Press, New York and London, pp 17–41
Bazylinski AD, Garratt-Reed JA, Frankel BR (1994) Electron microscopic studies of magnetosomes in magnetotactic bacteria. Microsc Res Tech 27(5):389–401. https://doi.org/10.1002/jemt.1070270505
Nakamura N, Matsunaga T (1993) Highly sensitive detection of allergen using bacterial magnetic particles. Anal Chim Acta 281:585–589. https://doi.org/10.1016/0003-2670(93)85018-F
Frankel RB, Bazylinski DA, Schueler D (1998) Biomineralization of magnetic iron minerals in bacteria. Supramol Sci 5:383–390. https://doi.org/10.1016/S0968-5677(98)00036-4
Winklhofer M, Petersen N (2000) Paleomagnetism and magnetic bacteria. Magnetoreception and magnetosomes in bacteria. Springer, Berlin Heidelberg, pp 255–273
Matsunaga T, MaedaY YT, Takeyama Harumi Ginya J, Aasahina T, Sakaguchi T, Tadokoro F (1991) Magnetite formation by a magnetic bacterium capable of growing aerobically. Appl Microbiol Biotechnol 35:651–655. https://doi.org/10.1007/BF00169632
Matsunaga T, Okamura Y (2002) Molecular mechanism of bacterial magnetite formation and its application. Mat Res Soc Symp Proc 724:11–24. https://doi.org/10.1557/PROC-724-N1.4
Revathy T, Jacob JJ, Jayasri MA, Suthindhiran K (2016) Isolation and characterization of Magnetospirillum from saline lagoon. World J Microbiol Biotechnol 32:109. https://doi.org/10.1007/s11274-016-2075-7
Sannigrahi S, Arumugasamy SK, Mathiyarasu J, Suthindhiran K (2020) Magnetosome-anti-Salmonella antibody complex based biosensor for the detection of Salmonella typhimurium. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2020.111071
Hameed AS, Yoganandhan K, Sathish S, Rasheed M, Murugan V, Jayaraman K (2001) White spot syndrome virus (WSSV) in two species of freshwater crabs (Paratelphusa hydrodomous and P. pulvinata). Aquaculture 201(3–4):179–186. https://doi.org/10.1016/S0044-8486(01)00525-7
Dinesh S, Karmarkar M, Vinodhini S, Vidhya G, Hemalatha K, Sudhakaran R (2014) Confirmation of Anti-WSSV activity from Red Algae Hypnae spinella in freshwater crab Paratelphusa hydrodomous. Int J ChemTech Res 6(8):4022–4026
Hameed AS, Balasubramanian G, Musthaq SS, Yoganandhan K (2003) Experimental infection of twenty species of Indian marine crabs with white spot syndrome virus (WSSV). Dis Aquat Org 57(1–2):157–161. https://doi.org/10.3354/dao057157
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Woo MA, Kim MI, Jung JH, Park KS, Seo TS, Park HG (2013) A novel colorimetric immunoassay utilizing the peroxidase mimicking activity of magnetic nanoparticles. Int J Mol Sci 14(5):9999–10014. https://doi.org/10.3390/ijms14059999
Talbot JP, Knobler LR, Buchmeier JM (1984) Western and dot immunoblotting analysis of viral antigens and antibodies: application to Murine Hepatitis virus. J Immunol Methods 73:177–188. https://doi.org/10.1016/0022-1759(84)90043-7
Reddy R, Bala C, Dinesh S, Anusha N, Itami T, Rajasekhara Reddy S, Sudhakaran R (2015) Antiviral activity of 3-(1-chloropiperidin-4-yl)-6-fluoro benzisoxazole 2 against White spot syndrome virus in Freshwater crab, Paratelphusa hydrodomous. Aquac Res 47(8):2677–2681. https://doi.org/10.1111/are.12704
Heyen U, Schüler D (2003) Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Appl Microbiol Biotechnol 61(5–6):536–544. https://doi.org/10.1007/s00253-002-1219-x
Jacob JJ, Suthindhiran K (2016) Magnetotactic bacteria and magnetosomes—scope and challenges. Mater Sci Eng C 68:919–928. https://doi.org/10.1016/j.msec.2016.07.049
Long RM, Dai QL, Zhou X, Cai DH, Hong YZ, Wang SB, Liu YG (2018) Bacterial magnetosomes-based nanocarriers for co-delivery of cancer therapeutics in vitro. Int J Nanomed 13:8269. https://doi.org/10.2147/IJN.S180503
Boucher M, Geffroy F, Prévéral S, Bellanger L, Selingue E, Adryanczyk-Perrier G, Pean M, Lefèvre CT, Pignol D, Ginet N, Mériaux S (2017) Genetically tailored magnetosomes used as MRI probe for molecular imaging of brain tumor. Biomaterials 121:167–178. https://doi.org/10.1016/j.biomaterials.2016.12.013
Zhang S, Moustafa Y, Huo Q (2014) Different interaction modes of biomolecules with citrate-capped gold nanoparticles. ACS Appl Mater Interfaces 6(23):21184–21192. https://doi.org/10.1021/am506112u
Auría-Soro C, Nesma T, Juanes-Velasco P, Landeira-Viñuela A, Fidalgo-Gomez H, Acebes-Fernandez V, Gongora R, Almendral Parra MJ, Manzano-Roman R, Fuentes M (2019) Interactions of nanoparticles and biosystems: microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials 9(10):1365. https://doi.org/10.3390/nano9101365
Lee Y, Lee S, Jon S (2018) Biotinylated bilirubin nanoparticles as a tumor microenvironment-responsive drug delivery system for targeted cancer therapy. Adv Sci 5(6):1800017. https://doi.org/10.1002/advs.201800017
Liu G, Lu M, Huang X, Li T, Xu D (2018) Application of gold-nanoparticle colorimetric sensing to rapid food safety screening. Sensors 18(12):4166. https://doi.org/10.3390/s18124166
Moghaddam AZ, Esmaeilkhanian E, Shakourian-Fard M (2019) Immobilizing magnetic glutaraldehyde cross-linked chitosan on graphene oxide and nitrogen-doped graphene oxide as well-dispersible adsorbents for chromate removal from aqueous solutions. Int J Biol Macromol 128:61–73. https://doi.org/10.1016/j.ijbiomac.2019.01.086
Liu BH, Lin YC, Ho CS, Yang CC, Chang YT, Chang JF, Li CY, Cheng CS, Huang JY, Lee YF, Hsu MH (2015) A novel detection platform for shrimp white spot syndrome virus using an ICP11-dependent immunomagnetic reduction (IMR) assay. PLoS ONE 10(9):e0138207. https://doi.org/10.1371/journal.pone.0138207
Józefczak A, Leszczyński B, Skumiel A, Hornowski T (2016) A comparison between acoustic properties and heat effects in biogenic (magnetosomes) and abiotic magnetite nanoparticle suspensions. J Magn Magn Mater 407:92–100. https://doi.org/10.1016/j.jmmm.2016.01.054
Arakaki A, Nakazawa H, Nemoto M, Mori T, Matsunaga T (2008) Formation of magnetite by bacteria and its application. J R Soc Interface 5(26):977–999. https://doi.org/10.1098/rsif.2008.0170
Lakshmipriya T, Gopinath SC, Hashim U, Tang TH (2016) Signal enhancement in ELISA: biotin-streptavidin technology against gold nanoparticles. J Taibah Univ Med Sci 11(5):432–438. https://doi.org/10.1016/j.jtumed.2016.05.010
Matsunaga T, Kamiya S (1987) Use of magnetic particles isolated from magnetotactic bacteria for enzyme immobilization. Appl Microbiol Biotechnol 26:328–332. https://doi.org/10.1007/BF00256663
Nakamura N, Burgess GJ, Yagiuda K, Kudo S, Sakaguchi T, Mataunaga T (1993) Detection and removal of Escherichia coli using fluorescein isothiocyanate conjugated monoclonal antibody immobilized on bacterial magnetic particles. Anal Chem 65:2036–2039. https://doi.org/10.1021/ac00063a018
Kulabhusan PK, Rajwade JM, Sugumar V, Taju G, Hameed AS, Paknikar KM (2017) Field-usable lateral flow immunoassay for the rapid detection of white spot syndrome virus (WSSV). PLoS ONE 12(1):e0169012. https://doi.org/10.1371/journal.pone.0169012
Loyprasert-Thananimit S, Saleedang A, Kanatharana P, Thavarungkul P, Chotigeat W (2012) Production of a polyclonal antibody to the VP26 nucleocapsid protein of White Spot Syndrome Virus (WSSV) and its use as a biosensor. Front Chem Sci Eng 6(2):216–223. https://doi.org/10.1007/s11705-012-1289-y
Pang S, Tsao HN, Feng X, Müllen K (2009) Patterned graphene electrodes from solution-processed graphite oxide films for organic field-effect transistors. Adv Mater 21(34):3488–3491. https://doi.org/10.1002/adma.200803812
Renedo OD, Alonso-Lomillo MA, Martinez MA (2007) Recent developments in the field of screen-printed electrodes and their related applications. Talanta 73(2):202–219. https://doi.org/10.1016/j.talanta.2007.03.050
Munteanu FD, Titoiu AM, Marty JL, Vasilescu A (2018) Detection of antibiotics and evaluation of antibacterial activity with screen-printed electrodes. Sensors 18(3):901. https://doi.org/10.3390/s18030901
Aalberse RC, Akkerdaas J, Van Ree R (2001) Cross-reactivity of IgE antibodies to allergens. Allergy 56(6):478–490. https://doi.org/10.1034/j.1398-9995.2001.056006478.x
Väkeväinen M, Eklund C, Eskola J, Käyhty H (2001) Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae evoked by pneumococcal conjugate vaccines, in infants. J Infect Dis 184(6):789–793. https://doi.org/10.1086/322984
Wu W, Wang L, Zhang X (2005) Identification of white spot syndrome virus (WSSV) envelope proteins involved in shrimp infection. Virology 332(2):578–583. https://doi.org/10.1016/j.virol.2004.12.011
Natarajan A, Devi KS, Raja S, Kumar AS (2017) An elegant analysis of white spot syndrome virus using a graphene oxide/methylene blue based electrochemical immunosensor platform. Sci Rep 7(1):1–11. https://doi.org/10.1038/srep46169(2017)
WSSV Detection Kit, http://geneilabs.com/product/wssv-detection-kit-25-tests/. Accessed 22 Mar 2021
Acknowledgements
This study was funded by a Grant-in-Aid from the Department of Biotechnology (DBT-No. BT/PR10570/PFN/20/839/2013).
Funding
Funded by the Department of Biotechnology (DBT-No. BT/PR10570/PFN/20/839/2013).
Author information
Authors and Affiliations
Contributions
SS performed the experiments and prepared the manuscript. SKA performed the electrochemical part, JM supervised the electrochemical part. RS supervised the VP28 extraction part. SK supervised the experiments described in the article. All authors approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
No humans or animals were used in this study.
Additional information
Handling Editor: Kalpana Agnihotri.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sannigrahi, S., Arumugasamy, S.K., Mathiyarasu, J. et al. Detection of white spot syndrome virus in seafood samples using a magnetosome-based impedimetric biosensor. Arch Virol 166, 2763–2778 (2021). https://doi.org/10.1007/s00705-021-05187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-021-05187-8