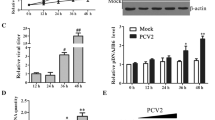
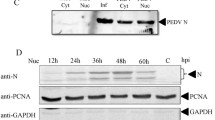
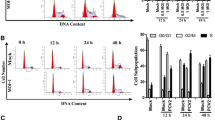
Abstract
Complement component 1 Q subcomponent-binding protein (C1QBP) has been shown to interact with the porcine circovirus type 2 (PCV2) Cap protein. Here, using yeast two-hybrid (Y2H) and co-immunoprecipitation assays, as well as laser confocal microscopy, the interaction between C1QBP and Cap was confirmed. Furthermore, overexpression of C1QBP in cells altered the intracellular location of Cap, which was observed using confocal microscopy and verified by detection of Cap in nuclear protein extracts in a Western blot assay. By inhibiting nuclear transport of Cap, overexpression of C1QBP downregulated PCV2 proliferation in PK-15 cells, as determined by quantitative polymerase chain reaction (qPCR). As C1QBP plays a similar role in a fusion of green fluorescent protein (GFP) with the Cap nuclear localisation signal (NLS) sequence, (CapNLS-GFP), we propose that the target site for C1QBP in Cap is possibly located in the NLS region. Considering all the results together, this study demonstrated that C1QBP interacts with the Cap NLS region, resulting in changes in the intracellular localisation of the Cap protein. We confirmed that overexpression of C1QBP inhibits the proliferation of PCV2, and this is possibly related to the function of C1QBP in controlling nuclear transport of Cap.







Similar content being viewed by others
References
Tischer I, Gelderblom H, Vettermann W, Koch MA (1982) A very small porcine virus with circular single-stranded DNA. Nature 295(5844):64
Hamel AL, Lin LL, Nayar GP (1998) Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J Virol 72(6):5262–5267
Meehan B, Mcneilly F, Todd D, Kennedy S, Jewhurst VA, Ellis J, Hassard L, Clark E, Haines DM, Allan GM (1998) Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J Gen Virol 79(9):2171–2179
Xiao CT, Halbur PG, Opriessnig T (2015) Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J Gen Virol 96(7):1830–1841
Giovanni F, Marti C, Joaquim S, Joseph H, Michele D (2016) Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol Phylogenet Evol 100:269–280
Xiao C-T, Harmon KM, Halbur PG, Opriessnig T (2016) PCV2d-2 is the predominant type of PCV2 DNA in pig samples collected in the U.S. during 2014–2016. Vet Microbiol 197:72–77
Carman S, Cai HY, Delay J, Youssef SA, Mcewen BJ, Gagnon CA, Tremblay D, Hazlett M, Lusis P, Fairles J (2009) The emergence of a new strain of porcine circovirus-2 in Ontario and Quebec swine and its association with severe porcine circovirus associated disease—2004–2006. Anim Sci Abroad 72(3):259
Wiederkehr DD, Sydler T, Buergi E, Haessig M, Zimmermann D, Pospischil A, Brugnera E, Sidler X (2009) A new emerging genotype subgroup within PCV-2b dominates the PMWS epizooty in Switzerland. Vet Microbiol 136(1–2):27–35
Tischer I, Bode L, Peters D, Pociuli S, Germann B (1995) Distribution of antibodies to porcine circovirus in swine populations of different breeding farms. Adv Virol 140(4):737–743
Lv Q-z, Guo K-k, Zhang Y-m (2014) Current understanding of genomic DNA of porcine circovirus type 2. Virus Genes 49(1):1–10
Opriessnig T, Meng X-J, Halbur PG (2007) Porcine circovirus type 2–associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest 19(6):591–615
Segalés J (2012) Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Re 164(1):1–19
Yin S, Sun S, Yang S, Shang Y, Cai X, Liu X (2010) Self-assembly of virus-like particles of porcine circovirus type 2 capsid protein expressed from Escherichia coli. Virol J 7(1):166
Liu Q, Tikoo SK, Babiuk LA (2001) Nuclear localization of the ORF2 protein encoded by porcine circovirus type 2. Virology 285(1):91–99
Hou Q, Hou S, Chen Q, Jia H, Xin T, Jiang Y, Guo X, Zhu H (2018) Nuclear localization signal regulates porcine circovirus type 2 capsid protein nuclear export through phosphorylation. Virus Res 246:12–22
Mo X, Li X, Yin B, Deng J, Tian K, Yuan A (2019) Structural roles of PCV2 capsid protein N-terminus in PCV2 particle assembly and identification of PCV2 type-specific neutralizing epitope. PLoS Pathog 15(3):e1007562
Marsh M, Helenius A (2006) Virus entry: open sesame. Cell 124(4):729–740
Steinfeldt T, Finsterbusch T, Mankertz A (2006) Demonstration of nicking/joining activity at the origin of DNA replication associated with the rep and rep′ proteins of porcine circovirus type 1. J Virol 80(13):6225–6234
Finsterbusch T, Steinfeldt T, Doberstein K, Rodner C, Mankertz A (2009) Interaction of the replication proteins and the capsid protein of porcine circovirus type 1 and 2 with host proteins. Virology 386(1):122–131
Hosszu KK, Santiagoschwarz F, Peerschke EIB, Ghebrehiwet B (2010) Evidence that a C1q/C1qR system regulates monocyte-derived dendritic cell differentiation at the interface of innate and acquired immunity. Innate Immun 16(2):115–127
Ghebrehiwet B, Geisbrecht BV, Xu X, Savitt AG, Peerschke EI (2019) The C1q receptors: focus on gC1qR/p33 (C1qBP, p32, HABP-1) 1. In: Seminars in immunology. Elsevier, Amsterdam, p 101338
Peerschke EIB, Ghebrehiwet B (2001) Human blood platelet gC1qR/p33. Immunol Rev 180(1):56–64
Peerschke EI, Minta JO, Zhou SZ, Bini A, Gotlieb A, Colman RW, Ghebrehiwet B (2004) Expression of gC1q-R/p33 and its major ligands in human atherosclerotic lesions. Mol Immunol 41(8):759–766
Gotoh K, Morisaki T, Setoyama D, Sasaki K, Yagi M, Igami K, Mizuguchi S, Uchiumi T, Fukui Y, Kang D (2018) Mitochondrial p32/C1qbp is a critical regulator of dendritic cell metabolism and maturation. Cell Rep 25(7):1800–1815
Uchiumi T, Kang D (2013) The functional and pathological analysis of mitochondrial protein p32 Rinsho byori. The Jpn J Clin Pathol 61(6):493–500
Jane Scully O, Yu Y, Salim A, Aye Thike A, Wai-Cheong Yip G, Hun Baeg G, Tan P-H, Matsumoto K, Huat Bay B (2015) Complement component 1, q subcomponent binding protein is a marker for proliferation in breast cancer. Exp Biol Med 240(7):846–853
Kim K, Kim MJ, Kim KH, Ahn SA, Kim JH, Cho JY, Yeo SG (2017) C1QBP is upregulated in colon cancer and binds to apolipoprotein AI. Exp Therap Med 13(5):2493–2500
Shi H, Fang W, Liu M, Fu D (2017) Complement component 1, q subcomponent binding protein (C1QBP) in lipid rafts mediates hepatic metastasis of pancreatic cancer by regulating IGF-1/IGF-1R signaling. Int J Cancer 141(7):1389–1401
Hu M, Li H-M, Bogoyevitch MA, Jans DA (2017) Mitochondrial protein p32/HAPB1/gC1qR/C1qbp is required for efficient respiratory syncytial virus production. Biochem Biophys Res Commun 489(4):460–465
Brudner M, Karpel M, Lear C, Chen L, Yantosca LM, Scully C, Sarraju A, Sokolovska A, Zariffard MR, Eisen DP (2013) Lectin-dependent enhancement of Ebola virus infection via soluble and transmembrane C-type lectin receptors. PLoS ONE 8(4):e60838
Bryant HE, Matthews DA, Wadd S, Scott JE, Kean J, Graham S, Russell WC, Clements JB (2000) Interaction between herpes simplex virus type 1 IE63 protein and cellular protein p32. J Virol 74(23):11322–11328
Du G, Stinski MF (2013) Interaction network of proteins associated with human cytomegalovirus IE2-p86 protein during infection: a proteomic analysis. PLoS ONE 8(12):e81583
Choi C-Y, Oh H-N, Lee SJ, Chun T (2015) ORF2 protein of porcine circovirus type 2 promotes phagocytic activity of porcine macrophages by inhibiting proteasomal degradation of complement component 1, q subcomponent binding protein (C1QBP) through physical interaction. J Gen Virol 96(11):3294–3301
Li D, Huang Y, Du Q, Wang Z, Chang L, Zhao X, Tong D (2016) CD40 ligand and GMCSF coexpression enhance the immune responses and protective efficacy of PCV2 adenovirus vaccine. Viral Immunol 29(3):148–158
Weingartl H, Sabara M, Pasick J, Van Moorlehem E, Babiuk L (2002) Continuous porcine cell lines developed from alveolar macrophages: partial characterization and virus susceptibility. J Virol Methods 104(2):203–216
Wang LS, Yin YB, Zou Y, Xi zhong HE, Zhao BJ, Chun hua LI (2013) Comparison between PCR and indirect immunofluorescence assay(IFA) methods for determining PCV2 TCID_(50). Acta Agriculturae Shanghai
Fu X, Gao X, He S, Huang D, Zhang P, Wang X, Zhang S, Dang R, Yin S, Du E (2013) Design and selection of a camelid single-chain antibody yeast two-hybrid library produced de novo for the cap protein of porcine circovirus type 2 (PCV2). PLoS ONE 8(3):e56222
Wang X, Lv C, Ji X, Wang B, Qiu L, Yang Z (2019) Ivermectin treatment inhibits the replication of Porcine circovirus 2 (PCV2) in vitro and mitigates the impact of viral infection in piglets. Virus Res 263:80–86
Changjie, Wenkai, Liu, Bin, Wang, Ruyi, Dang, Qiu, Juan, Ren (2018) Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res
Cao J, Lin C, Wang H, Wang L, Zhou N, Jin Y, Liao M, Zhou J (2015) Circovirus transport proceeds via direct interaction of the cytoplasmic dynein IC1 subunit with the viral capsid protein. J Virol 89(5):2777–2791
Lowe AR, Tang JH, Yassif J, Graf M, Huang WY, Groves JT, Weis K, Liphardt JT (2015) Importin-β modulates the permeability of the nuclear pore complex in a Ran-dependent manner. Elife 4:e04052
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31672581).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling Editor: Akbar Dastjerdi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, X., Lv, C., Wang, Q. et al. C1QBP inhibits proliferation of porcine circovirus type 2 by restricting nuclear import of the capsid protein. Arch Virol 166, 767–778 (2021). https://doi.org/10.1007/s00705-020-04950-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-020-04950-7