Abstract
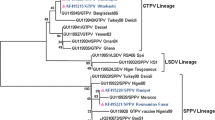
Sheeppox and goatpox are important transboundary animal viral diseases of sheep and goats caused by sheeppox virus (SPPV) and goatpox virus (GTPV), respectively, of the genus Capripoxvirus, family Poxviridae. Among the proteins encoded by the capripoxvirus (CaPV) genome, ORF095 (vaccinia virus A4L homolog) is an immunodominant virion core protein that plays a pivotal role in virus assembly and morphogenesis. In the present study, sequence analysis of the ORF095 genes of 27 SPPV and GTPV isolates or field samples from different geographical regions of India was performed, and structure was prediction was done by homology modeling. A multiple sequence alignment of different CaPV isolates revealed that CaPV-A4L is highly conserved, with several species-specific signature residues, namely A93, A216, A315, G136 and G146 in GTPV, G47, A63, A168 and A276 in SPPV, and G48 and C98 in lumpy skin disease virus (LSDV). Phylogenetically, the CaPV isolates were separated into three major clusters, GTPV, SPPV and LSDV, based on the complete coding sequence of the CaPV-A4L gene. Genus-specific clustering of poxviruses was observed in phylogenetic analysis based on A4L protein homologs of chordopoxviruses. A secondary structure prediction showed the presence of six α-helices and one β-sheet as well as some coils. The signature residues identified here are potentially useful for genotyping, and the predicted characteristics of the CaPV-A4L protein make it an ideal candidate for use as an immunogenic or diagnostic antigen for the development of immunoassays in the sero-evaluation of CaPV in target hosts.





Similar content being viewed by others
References
International Committee on Taxonomy of Viruses (ICTV) https://talk.ictvonline.org/taxonomy/
Office Internationale des epizooties (World Health Organization for animals) WAHIS portal: animal health data. https://www.oie.int/en/animal-health-in-the-world/wahis-portal-animal-health-data
Madhavan A, Venkatesan G, Kumar A (2016) Capripoxviruses of small ruminants: current updates and future perspectives. Asian J Anim Vet Adv 11:757–770
Garner MG, Sawarkar SD, Brett EK, Edwards JR, Kulkarni VB, Boyle DB, Singh SN (2000) The extent and economic impact of sheep pox and goat pox in the state of Maharashtra, India. Trop Anim Health Prod 32:205–223
Yeruham I, Yadin H, Van Ham M, Bumbarov V, Soham A, Perl S (2007) Economic and epidemiological aspects of an outbreak of sheeppox in a dairy sheep flock. Vet Rec 160(7):236–237
Babiuk S, Bowden TR, Boyle DB, Wallace DB, Kitching RP (2008) Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transbound Emerg Dis 55:263–272
Bhanuprakash V, Venkatesan G, Balamurugan V, Hosamani M, Yogisharadhya R, Chauhan RS, Pande A, Mondal B, Singh RK (2010) Pox outbreaks in sheep and goats at Makhdoom (Uttar Pradesh), India: evidence of sheep pox virus infection in goats. Transbound Emerg Dis 57:375–382
Bhanuprakash V, Hosamani M, Singh RK (2011) Prospects of control and eradication of capripox from the Indian subcontinent: a perspective. Antivir Res 91:225–232
Hosamani M, Mondal B, Tembhurne PA, Bandyopadhyay SK, Singh RK, Rasool TJ (2004) Differentiation of sheep pox and goat poxviruses by sequence analysis and PCR-RFLP of P32 gene. Virus Genes 29:73–80
Venkatesan G, Balamurugan V, Yogisharadhya R, Kumar A, Bhanuprakash V (2012) Differentiation of sheeppox and goatpox viruses by polymerase Chain reaction-restriction fragment length polymorphism. Virol Sin 27(6):353–359
Tulman ER, Afonso CL, Lu Z, Zsak L, Sur JH, Sandybaev NT, Kerembekova UZ, Zaitsev VL, Kutish GF, Rock DL (2002) The genomes of sheeppox and goatpox viruses. J Virol 76(12):6054–6061
Zhao Z, Wu G, Zhu X, Yan X, Dou Y, Li J, Zhu H, Zhang Q, Cai X (2012) RNA interference targeting virion core protein ORF095 inhibits goatpox virus replication in Vero cells. Virol J 9:48
Maa JS, Esteban M (1987) Structural and functional studies of a 39,000-Mr immunodominant protein of vaccinia virus. J Virol 61:3910–3919
Bowden TR, Coupar BE, Babiuk SL, White JR, Boyd V, Duch CJ, Shiell BJ, Ueda N, Parkyn GR, Copps JS, Boyle DB (2009) Detection of antibodies specific for sheeppox and goatpox viruses using recombinant capripoxvirus antigens in an indirect enzyme-linked immunosorbent assay. J Virol Methods 161(1):19–29
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Jones DT (1999) PSIPRED: protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 292(2):195–202
Lupas A, Van Dyke M, Stock J (1991) Predicting coiled coils from protein sequences. Science 252:1162–1164
Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinform 9:40
Yang J, Zhang Y (2015) I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res 43:W174–W181
Tuppurainen ES, Pearson CR, Bachanek-Bankowska K, Knowles NJ, Amareen S, Frost L, Henstock MR, Lamien CE, Diallo A, Mertens PP (2014) Characterization of sheep pox virus vaccine for cattle against lumpy skin disease virus. Antivir Res 109:1–6
Santhamani R, Venkatesan G, Minhas SK, Shivachandra SB, Muthuchelvan D, Pandey AB, Ramakrishnan MA (2015) Detection and characterization of atypical capripoxviruses among small ruminants in India. Virus Genes 51(1):33–38
Lamien CE, Le Goff C, Silber R, Wallace DB, Gulyaz V, Tuppurainen E, Madani H, Caufour P, Adam T, El Harrak M, Luckins AG, Albina E, Diallo A (2011) Use of the Capripoxvirus homologue of Vaccinia virus 30 kDa RNA polymerase subunit (RPO30) gene as a novel diagnostic and genotyping target: development of a classical PCR method to differentiate Goatpoxvirus from Sheep poxvirus. Vet Microbiol 149:30–39
Venkatesan G, Balamurugan V, Bhanuprakash V (2014) Multiplex PCR for simultaneous detection and differentiation of sheeppox, goatpox and orf viruses from clinical samples of sheep and goats. J Virol Methods 195:1–8
Greth A, Gourreau JM, Vassart M, Nguyen-Ba-Vy, Wyers M, Lefevre PC (1992) Capripoxvirus disease in an Arabian oryx (Oryx leucoryx) from Saudi Arabia. J Wildl Dis 28(2):295–300
Zeng X, Chi X, Li W, Hao W, Li M, Huang X, Huang Y, Rock DL, Luo S, Wang S (2014) Complete genome sequence analysis of goatpox virus isolated from China shows high variation. Vet Microbiol 173(1–2):38–49
Karki M, Kumar A, Venkatesan G, Arya S, Pandey AB (2018) Genetic analysis of L1R myristoylated protein of Capripoxviruses reveals structural homogeneity among poxviruses. Infect Genet Evol 58:224–231
Le Goff C, Lamien CE, Fakhfakh E, Chadeyras A, Aba-Adulugba E, Libeau G, Tuppurainen E, Wallace DB, Adam T, Silber R, Gulyaz V, Madani H, Caufour P, Hammami S, Diallo A, Albina E (2009) Capripoxvirus G-protein-coupled chemokine receptor: a host-range gene suitable for virus animal origin discrimination. J Gen Virol 90(8):1967–1977
Van Vliet K, Mohamed MR, Zhang L, Villa NY, Werden SJ, Liu J, McFadden G (2009) Poxvirus proteomics and virus-host protein interactions. Microbiol Mol Biol Rev 73:730–749
Demkowicz WE, Maa JS, Esteban M (1992) Identification and characterization of vaccinia genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J Virol 66:386–398
Cudmore S, Blasco R, Vincentelli R, Esteban M, Sodeik B, Griffithsm G, Locker JK (1996) A vaccinia virus core protein, p39, is membrane associated. J Virol 70:6909–6921
Boulanger D, Green P, Smith T, Czerny CP, Skinner MA (1998) The 131-amino-acid repeat region of the essential 39-kilodalton core protein of fowlpox virus FP9, equivalent to vaccinia virus A4L protein, is nonessential and highly immunogenic. J Virol 72(1):170–179
Chang T, Chang S, Hsieh F, Ko T, Lin C, Ho MR, Wang I, Hsu ST, Guo RT, Chang W, Wang AH (2013) Crystal structure of vaccinia viral A27 protein reveals a novel structure critical for its function and complex formation with A26 protein. PLoS Pathog 9:e1003563
Kumar A, Yogisharadhya R, Bhanuprakash V, Venkatesan G, Shivachandra SB (2015) Structural analysis and immunogenicity of recombinant major envelope protein (rA27L) of buffalpox virus, a zoonotic Indian vaccinia-like virus. Vaccine 33(41):5396–5405
Dashprakash M, Venkatesan G, Kumar A, Sankar M, Arya S, Ramakrishnan MA, Pandey AB, Mondal B (2019) Prokaryotic expression, purification and evaluation of goatpox virus ORF117 protein as a diagnostic antigen in indirect ELISA to detect goatpox. Arch Virol 164(4):1049–1058
Mathijs E, Vandenbussche F, Haegeman A, Al-Majali A, De Clercq K, Van Borm S (2016) Complete genome sequence of the goatpox virus strain Gorgan obtained directly from a commercial live attenuated vaccine. Genome Announc 4(5)
Kara PD, Afonso CL, Wallace DB, Kutish GF, Abolnik C, Lu Z, Vreede FT, Taljaard LC, Zsak A, Viljoen GJ, Rock DL (2003) Comparative sequence analysis of the South African vaccine strain and two virulent field isolates of Lumpy skin disease virus. Arch Virol 148(7):1335–1356
Tulman ER, Afonso CL, Lu Z, Zsak L, Kutish GF, Rock DL (2001) Genome of lumpy skin disease virus. J Virol 75:7122–7130
Mathijs E, Vandenbussche F, Haegeman A, King A, Nthangeni B, Potgieter C, Maartens L, Van Borm S, De Clercq K (2016) Complete genome sequences of the Neethling-like lumpy skin disease virus strains obtained directly from three commercial live attenuated vaccines. Genome Announc 4(6):e01255–16
Agianniotaki EI, Mathijs E, Vandenbussche F, Tasioudi KE, Haegeman A, Iliadou P, De Clercq K (2017) Complete genome sequence of the lumpy skin disease virus isolated from the first reported case in Greece in 2015. Genome Announc 5(29)
Vandenbussche F, Mathijs E, Haegeman A, Al-Majali A, Van Borm S, De Clercq K (2016) Complete genome sequence of Capripoxvirus strain KSGP 0240 from a commercial live attenuated vaccine. Genome Announc 4(5):e01114–e01116
Toplak I, Petrovic T, Vidanovic D, Lazic S, Sekler M, Manic M, Kuhar U (2017) Complete genome sequence of lumpy skin disease virus isolate SERBIA/Bujanovac/2016, Detected during an outbreak in the Balkan Area. Genome Announc 5(35)
Lojkic I, Simic I, Kresic N, Bedekovic T (2018) Complete genome sequence of a lumpy skin disease virus strain isolated from the skin of a vaccinated animal. Genome Announc 6(22).
Acknowledgements
The authors thank the Director, Indian Veterinary Research Institute, for providing necessary facilities to carry out this work, and the staff of the pox virus laboratory, IVRI, Mukteswar, for their valuable and timely help in carrying out this work. The authors also thank the Directors and staff of the regional disease diagnostic laboratories of the state animal husbandry departments for sending the clinical samples to the pox laboratory, IVRI, Mukteswar, for diagnostic investigation of animal viral diseases. This study is part of the MVSc thesis work of the first author. The financial support provided by Indian Veterinary Research Institute, Izatnagar, under institute project (IXX09899) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Handling Editor: William G Dundon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Madhavan, A., Venkatesan, G., Kumar, A. et al. Comparative sequence and structural analysis of the ORF095 gene, a vaccinia virus A4L homolog of capripoxvirus in sheep and goats. Arch Virol 165, 1419–1431 (2020). https://doi.org/10.1007/s00705-020-04623-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-020-04623-5