Abstract
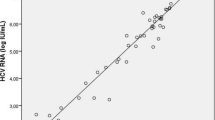
HCV is a potential cause of viral hepatitis, which leads to blood-borne chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. Anti-HCV antibody detection assays detect HCV infection after approximately 70 days. HCV core antigen can be detected much earlier than anti-HCV antibodies. However, it disappears soon after the appearance of anti-HCV antibodies. Thus, there is a need for a rapid assay for simultaneous detection of HCV core antigen and anti-HCV antibodies for early diagnosis of HCV infection. A rapid diagnostic assay (HCV Ag-Ab Combo) for simultaneous detection of HCV core antigen and anti-HCV antibodies for early diagnosis of HCV infection was developed. HCV Ag-Ab Combo was evaluated in order to determine its potential for detection of HCV infection earlier than anti-HCV antibody detection assays. We compared the sensitivity of the newly developed assay with anti-HCV antibody detection assays (ELISA [HCV Ab ELISA] and rapid test [HCV Ab Rapid]) and HCV core antigen/anti-HCV antibody detection ELISA (HCV Ag-Ab ELISA). This study included 11 samples that were found positive in HCV RNA detection and HCV Ag-Ab ELISA but negative in HCV antibody detection assays (HCV Ab ELISA and rapid), 10 samples that were found positive in HCV Ag-Ab ELISA and HCV Ab ELISA but negative in HCV Ab Rapid, 69 samples that were found positive in HCV Ag-Ab ELISA, HCV Ab ELISA, and HCV Ab Rapid, and 509 samples that were found negative in HCV Ag-Ab ELISA, HCV Ab ELISA, and HCV Ab Rapid. Three seroconversion panels, PHV 913, PHV 911 (M) and PHV904-00-1.0, and a HCV RNA genotype qualification panel (2400-0182) acquired from Seracare Life Sciences (USA) were also tested. HCV Ag-Ab Combo showed a combined sensitivity and specificity of 100% when tested with 90 samples that were positive for HCV by HCV Ag-Ab ELISA and 509 HCV-negative samples. Its positive predictive value (PPV) and negative predictive value (NPV) were found to be 100%. It detected HCV infection approximately 7 to 12 days earlier than the HCV Ab detection assays, and its performance was not affected when testing different genotypes of HCV. HCV Ag-Ab Combo did not detect HCV infection as early as HCV RNA or HCV Ag-Ab ELISA. HCV Ag-Ab Combo provided a significant improvement for the early detection of HCV infection during the preseroconversion phase when compared with anti-HCV antibody detection assays. It could be a useful screening assay, and an alternative to HCV RNA detection or HCV Ag-Ab ELISA when nucleic acid technologies cannot be implemented.





Similar content being viewed by others
References
Alavian SM, Adibi P, Zali MR (2005) Hepatitis C virus in Iran: epidemiology of an emerging infection. Arch Iran Med 8(2):84–90
Alter MJ (2007) Epidemiology of Hepatitis C virus infection. World J Gastroenterol 13(17):2436–2441
WHO (2017) Global hepatitis report. https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Accessed 17 May 2019
Rafik M, Bakr S, Soliman D, Mohammed N, Ragab D, ElHady WA, Samir N (2016) Characterization of differential antibody production against Hepatitis C virus in different HCV infection status. Virol J 13:116
Hayashi N, Takehara T (2006) Antiviral therapy for chronic Hepatitis C: past, present, and future. J Gastroenterol 41:17–27
Al-Nassarya MSK, Mahdib BM (2018) Study of Hepatitis C virus detection assays. Ann Med Surg 36:47–50
Barban V, Fraysse-Corgier S, Paranhos-Baccala G, Petit M, Manin C, Berard Y, Prince AM, Mandrand B, Meulien P (2000) Identification of a human epitope in Hepatitis C virus (HCV) core protein using a molecularly cloned antibody repertoire from a non-symptomatic, anti-HCV-positive patient. J Gen Virol 81:461–469
Kalliampakou K, Kalamvoki M, Mavromara P (2005) Hepatitis C virus (HCV) NS5A protein down regulates HCV IRES-dependent translation. J Gen Virol 86:1015–1025
Ali A, Nisar M, Idrees M, Rafique S, Iqbal M (2015) Expression of Hepatitis C virus core and E2 antigenic recombinant proteins and their use for development of diagnostic assays. Int J Infect Dis 34:84–89
Beld M, Penning M, Putten MV, Lukashov V, Hoek AVD, Mcmorrow M, Goudsmit J (1999) Quantitative antibody responses to structural (core) and nonstructural (NS3, NS4, and NS5) Hepatitis C virus proteins among seroconverting injecting drug users: impact of epitope variation and relationship to detection of HCV RNA in blood. Hepatology 29(4):1288–1298
Icardi G, Ansaldi F, Bruzzone BM, Durando P, Lee S, Luigi C, Crovari P (2001) Novel approach to reduce the Hepatitis C virus (HCV) window period: clinical evaluation of a new enzyme-linked immunosorbent assay for HCV core antigen. J Clin Microbiol 39(9):3110–3114
El-Emshaty WM, Raafat D, Doaa M, Elghannam DM, Saudy N, Eltoraby EE, Metwalli EA (2011) Diagnostics performance of an immuno assay for simultaneous detection of HCV core antigen and antibodies among haemodialysis patients. Braz J Microbiol 42:303–309
National Institutes of Health Consensus Development Conference Panel statement (1997) Management of Hepatitis C. Hepatology 26(3 Suppl 1):2S–10S
Marshall DA, Kleinman SH, Wong JB, AuBuchon JP, Grima DT, Kulin NA, Weinstein MC (2004) Cost-effectiveness of nucleic acid test screening of volunteer blood donations for Hepatitis B, Hepatitis C and human immunodeficiency virus in the United States. Vox Sang 86(1):28–40
Bouvier-Alias M, Patel K, Dahari H, Beaucourt S, Larderie P, Blatt L, Hezode C, Picchio G, Dhumeaux D, Neumann A, McHutchison J, Pawlotsky JM (2002) Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology 36(1):211–218
Cresswell F, Fisher M, Hughes D, Shaw S, Homer G, Hassan-Ibrahim M (2015) Hepatitis C core antigen testing: a reliable, quick, and potentially cost-effective alternative to Hepatitis C polymerase chain reaction in diagnosing acute Hepatitis C virus infection. Clin Infect Dis 60(2):263–266
Dawson G (2012) The potential role of HCV core antigen testing in diagnosing HCV infection. Antivir Ther 17:1431–1435
Gaudy C, Thevenas C, Tichet J, Mariotte N, Goudeau A, Dubois F (2005) Usefulness of the Hepatitis C virus core antigen assay for screening of a population undergoing routine medical checkup. J Clin Microbiol 43(4):1722–1726
Kim MN, Kim HS, Kim JK, Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, Han KH (2016) Clinical utility of a new automated Hepatitis C virus core antigen assay for prediction of treatment response in patients with chronic Hepatitis C. J Korean Med Sci 31:1431–1437
Massaguer A, Forns X, Costa J, Feliu A, Garcıa-Retortillo M, Navasa M, Rimola A, Garcia-Valdecasas JC, Sanchez-Tapias JM (2005) Performance of Hepatitis C virus core antigen immunoassay in monitoring viral load after liver transplantation. Transplantation 79:1441–1444
Pivert A, Payan C, Morand P, Fafi-Kremer S, Deshayes J, Carrat F, Pol S, Cacoub P, Perronne C, Lunel F (2006) Comparison of serum Hepatitis C virus (HCV) RNA and core antigen levels in patients coinfected with human immunodeficiency virus and HCV and treated with interferon plus ribavirin. J Clin Microbiol 44(2):417–422
Murayama A, Sugiyama N, Watashi K, Masaki T, Suzuki R, Aizaki H, Mizuochi T, Wakita T, Kato T (2012) Japanese reference panel of blood specimens for evaluation of Hepatitis C virus RNA and core antigen quantitative assays. J Clin Microbiol 50(6):1943–1949
Ross RS, Viazov S, Salloum S, Hilgard P, Gerken G, Roggendorf M (2010) Analytical performance characteristics and clinical utility of a novel assay for total Hepatitis C virus core antigen quantification. J Clin Microbiol 48(4):1161–1168
Reddy AK, Dakshinamurty KV, Lakshmi V (2006) Utility of HCV core antigen ELISA in the screening for Hepatitis C virus infection in patients on hemodialysis. Indian J Med Microbiol 24(1):55–57
Laperche S, Marrec NL, GiraultA Bouchardeau F, Servant-Delmas A, Maniez-Montreuil M, Gallian P, Levayer T, Morel P, Simon N (2005) Simultaneous detection of Hepatitis C virus (HCV) core antigen and anti-HCV antibodies improves the early detection of HCV infection. J Clin Microbiol 43(8):3877–3883
Patel J, Sharma P (2017) Determination of potential of HCV mosaic protein for the diagnosis of Hepatitis C virus infection. Int J Adv Sci Eng Technol 5(3):63–67
Chan HKW, Theolis R (2002) HCV mosaic antigen composition, Patent WO 2,002,004,484 A2
He Lishan, Nan Tiegui, Cui Yongliang, Guo Suqin, Zhang Wei, Zhang Rui, Tan Guiyu, Wang Baomin, Cui Liwang (2014) Development of a colloidal gold-based lateral flow dipstick immunoassay for rapid qualitative and semi-quantitative analysis of artesunate and dihydroartemisinin. Malar J 13:127
Maheshwari A, Ray S, Thuluvath PJ (2008) Acute hepatitis C infection. Lancet 372:321–332
Glenn SA, Wright DJ, Klienman SH, Hirschkorn D, Tu Y, Helderbrant C (2005) Dynamics of viremia in early Hepatitis C virus infection. Transfusion 45:994–1002
Kamal SM (2008) Acute Hepatitis C: a systematic review. Am J Gastroenterol 103:1283–1297
Wang L, Hong LV, Zhang G (2016) Hepatitis C virus core antigen assay: an alternative method for Hepatitis C diagnosis. Ann Clin Biochem 0(0):1–7
Marwaha N, Sachdev S (2014) Current testing strategies for Hepatitis C virus infection in blood donors and the way forward. World J Gastroenterol 20(11):2948–2954
Courouce AM, Le Marrec N, Bouchardeau F, Razer A, Ma-niez M, Laperche S, Simon N (2000) Efficacy of HCVcore antigen detection during the preseroconversion period. Transfusion 40:1198–1202
Grant PR, Sims CM, Tedder RS (2002) Quantification of HCVRNA levels and detection of core antigen in donations before sero-conversion. Transfusion 42:1032–1036
Peterson J, Green G, Iida K, Caldwell B, Kerrison P, Bernich S, Aoyagi K, Lee SR (2000) Detection of Hepatitis C core antigen in the antibody negative ‘window’ phase of Hepatitis C infection. Vox Sang 78:80–85
Desbois D, VaghefiP Savary J, Dussaix E, Roque-Afonso A (2008) Sensitivity of a rapid immuno-chromatographic test for Hepatitis C antibodies detection. J Clin Virol 41:129–133
Hassaneina F, Shehata AI (2018) Rapid immunochromatographic test (RDT) versus ELISA technique for diagnosing Toxoplasmosis among individuals with mental disabilities. Int J Innov Res Dev 7(6):61–66
Cividini A, Cerino A, Muzzi Alba, Furione Milena, Rebucci Chiara, Segagni Laura, Gatti Marta, Barnaba Vincenzo, Mondelli Mario U (2003) kinetics and significance of serum Hepatitis C virus core antigen in patients with acute Hepatitis C. J Clin Microbiol 41:2144–2146
Hadziyannis Emilia, Minopetrou Martha, Georgiou Anastasia, Spanou Fotini, Koskinas John (2013) Is HCV core antigen a reliable marker of viral load? An evaluation of HCV core antigen automated immunoassay. Ann Gastroenterol 26:146–149
Krajden M, Shivji R, Gunadasa K, McNabb G, Mak A, McNabb G, Friesenhahn M, Hendricks D, Comanor L (2004) Evaluation of the core antigen assay as a second-line supplemental test for diagnosis of active Hepatitis C virus infection. J Clin Microbiol 42(9):4054–4059
Acknowledgements
The authors acknowledge the Director of R&D, Arkray Healthcare Pvt. Ltd., for providing resources and facilities to carry out the study.
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Ethical approval
The work was carried out with the permission of the ethical committees of the institutions.
Additional information
Handling Editor: Ioly Kotta-Loizou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, J., Sharma, P. Design of a novel rapid immunoassay for simultaneous detection of hepatitis C virus core antigen and antibodies. Arch Virol 165, 627–641 (2020). https://doi.org/10.1007/s00705-019-04518-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04518-0