Abstract
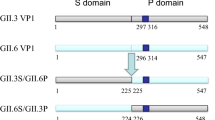
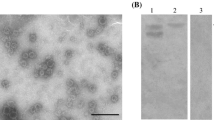
Trypsin digestion promotes disassembly of GII.3 NoV virus-like particles (VLPs) and binding of VLPs to salivary histo-blood group antigens (HBGAs), but it is not clear which specific regions or residues mediate viral attachment to HBGAs. An earlier study indicated that arginine residues in the predicted surface-exposed loop region are susceptible to trypsin digestion. Here, we introduced single or multiple substitutions of four arginine residues located in the predicted surface-exposed loop region of the GII.3 NoV capsid protein (VP1) and observed their effects on susceptibility to trypsin digestion and binding to HBGAs. All of the mutations in VP1, including single substitutions (R287G, R292G, R296G or R307G) and quadruple substitutions (R287G, R292G, R296G and R307G), permitted successful VLP assembly. After tryptic digestion, all VP1 proteins bearing single point mutations were cleaved, resulting in complete digestion or single fragments with various molecular sizes (27-35 kDa), while the VP1 protein bearing four substitutions was cleaved into two fragments (27-55 kDa). Binding assays using synthetic and salivary HBGAs showed that none of the VP1 mutants (singly or quadruply substituted) exhibited detectable binding to HBGA before or after trypsin cleavage. These results indicated that arginine residues within the predicted surface loop region of GII.3 NoV VP1 were involved directly or indirectly in binding salivary HBGAs and could potentially mediate the HBGA-GII.3 NoV interactions through which host cells become infected.




Similar content being viewed by others
References
Prasad B, Rothnagel R, Jiang X, Estes M (1994) Three-dimensional structure of baculovirus-expressed Norwalk virus capsids. J Virol 68(8):5117–5125
Prasad B, Hardy M, Dokland T, Bella J, Rossmann M, Estes M (1999) X-ray crystallographic structure of the Norwalk virus capsid. Science 286(5438):287–290
Tan M, Hegde R, Jiang X (2004) The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J Virol 78(12):6233–6242
Vinjé J (2015) Advances in laboratory methods for detection and typing of norovirus. J Clin Microbiol 53(2):373–381
Donaldson E, Lindesmith L, Lobue A, Baric R (2008) Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol Rev 225:190–211
van Beek J, de Graaf M, Al-Hello H, Allen D, Ambert-Balay K, Botteldoorn N et al (2018) Molecular surveillance of norovirus, 2005–16: an epidemiological analysis of data collected from the NoroNet network. Lancet Infect Dis 18(5):545–553
Chan M, Leung T, Chung T, Kwok A, Nelson E, Lee N et al (2015) Virus genotype distribution and virus burden in children and adults hospitalized for norovirus gastroenteritis, 2012–2014. Hong Kong Sci Rep 5:11507
Bucardo F, Reyes Y, Becker-Dreps S, Bowman N, Gruber J, Vinjé J et al (2017) Pediatric norovirus GII.4 infections in Nicaragua, 1999–2015. Infect Genet Evol 55:305–312
Boon D, Mahar J, Abente E, Kirkwood C, Purcell R, Kapikian A et al (2011) Comparative evolution of GII.3 and GII.4 norovirus over a 31-year period. J Virol 85(17):8656–8666
Mahar J, Bok K, Green K, Kirkwood C (2013) The importance of intergenic recombination in norovirus GII3 evolution. J Virol. 87(7):3687–3698
Ayouni S, Estienney M, Sdiri-Loulizi K, Ambert-Balay K, de Rougemont A, Aho S et al (2015) Relationship between GII.3 norovirus infections and blood group antigens in young children in Tunisia. Clin Microbiol Infect 21(9):874.e1–878.e1
Ettayebi K, Crawford S, Murakami K, Broughman J, Karandikar U, Tenge V et al (2016) Replication of human noroviruses in stem cell-derived human enteroids. Science 353(6306):1387–1393
Huo Y, Wan X, Ling T, Shen S (2016) Biological and immunological characterization of norovirus major capsid proteins from three different genotypes. Microb Pathog 90:78–83
Almand EA, Moore MD, Jaykus LA (2017) Norovirus binding to ligands beyond histo-blood group antigens. Front Microbiol 8:2549
Huo Y, Wang W, Zheng L, Chen X, Shen S, Wang M (2017) Enzymatic cleavage promotes disassembly of GII.3 norovirus virus like particles and its binding to salivary histo-blood group antigens. Virus Res 240:18–24
Kumar S, Ochoa W, Kobayashi S, Reddy V (2007) Presence of a surface-exposed loop facilitates trypsinization of particles of Sinsiro virus, a genogroup II3 norovirus. J Virol 81(3):1119–1128
Liu J, Li S, Wang C, Zheng L, Ma J, Li C et al (2018) Genomic characterization of GII.3 noroviruses isolated from children in Zhengzhou city, China, 2015/16. Arch Virol
Bertolotti-Ciarlet A, Crawford S, Hutson A, Estes M (2003) The 3’ end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J Virol 77(21):11603–11615
Zheng L, Wang W, Liu J, Chen X, Li S, Wang Q et al (2018) Characterization of a Norovirus-specific monoclonal antibody that exhibits wide spectrum binding activities. J Med Virol 90(4):671–676
Koho T, Mäntylä T, Laurinmäki P, Huhti L, Butcher S, Vesikari T et al (2012) Purification of norovirus-like particles (VLPs) by ion exchange chromatography. J Virol Methods 181(1):6–11
Huo Y, Wan X, Wang Z, Meng S, Shen S (2015) Production of Norovirus VLPs to size homogeneity. Virus Res 204:1–5
White L, Hardy M, Estes M (1997) Biochemical characterization of a smaller form of recombinant Norwalk virus capsids assembled in insect cells. J Virol 71(10):8066–8072
Pogan R, Schneider C, Reimer R, Hansman G, Uetrecht C (2018) Norovirus-like VP1 particles exhibit isolate dependent stability profiles. J Phys Condens Matter Inst Phys J 30(6):064006
Katayama K, Murakami K, Sharp T, Guix S, Oka T, Takai-Todaka R et al (2014) Plasmid-based human norovirus reverse genetics system produces reporter-tagged progeny virus containing infectious genomic RNA. Proc Natl Acad Sci USA 111(38):E4043–E4052
Kilic T, Koromyslova A, Hansman GS (2019) Structural basis for human norovirus capsid binding to bile acids. J Virol 93(2):1581
Nelson CA, Wilen CB, Dai YN, Orchard RC, Kim AS, Stegeman RA, Hsieh LL, Smith TJ, Virgin HW, Fremont DH et al (2018) Structural basis for murine norovirus engagement of bile acids and the CD300lf receptor. Proc Natl Acad Sci USA 115(39):E9201–E9210
Kilic T, Koromyslova A, Malak V, Hansman GS (2018) Atomic structure of the murine norovirus protruding domain and soluble CD300lf receptor complex. J Virol 92(11):413–418
Acknowledgements
This work was supported by the Initiation Fund setup program for newly enrolled doctoral personnel at The Sixth People’s Hospital of Zhengzhou. We thank the Core Facility and Technical Support of Wuhan Institute of Virology, CAS, for their technical assistance in fluorescence microscopy (Ding Gao and Juan Min) and electron microscopy (Pei Zhang, Anna Du and Bichao Xu).
Funding
YH and XS conceived and designed the experiments. GZ, JL, LZ, JW, WW performed the experiments. GZ and YH analyzed the data and prepared the manuscript. YH and XS revised the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Reimar Johne.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, G., Wang, J., Liu, J. et al. The surface-exposed loop region of norovirus GII.3 VP1 plays an essential role in binding histo-blood group antigens. Arch Virol 164, 1629–1638 (2019). https://doi.org/10.1007/s00705-019-04256-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04256-3