Abstract
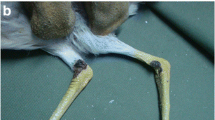
Infections caused by mule deerpox virus (MDPV) have been sporadically reported in North American cervids. White-tailed deer (Odocoileus virginianus) fawns from a farm located in South Central Florida presented with ulcerative and crusting lesions on the coronary band as well as the mucocutaneous tissues of the head. Evaluation of the crusted skin lesions was undertaken using microscopic pathology and molecular techniques. A crusted skin sample was processed for virus isolation in four mammalian cell lines. The resulting isolate was characterized by negative staining electron microscopy and deep sequencing. Histopathologic evaluation of the skin lesions from the fawns revealed a hyperplastic and proliferative epidermis with ballooning degeneration of epidermal and follicular keratinocytes with intracytoplasmic eosinophilic inclusions. Electron microscopy of cell culture supernatant demonstrated numerous large brick-shaped particles typical of most poxviruses. Polymerase chain reaction assays followed by Sanger sequencing revealed a poxvirus gene sequence nearly identical to that of previous strains of MDPV. The full genome was recovered by deep sequencing and genetic analyses supported the Florida white-tailed deer isolate (MDPV-F) as a strain of MDPV. Herein, we report the first genome sequence of MDPV from a farmed white-tailed deer fawn in the South Central Florida, expanding the number of locations and geographic range in which MDPV has been identified.






Similar content being viewed by others
References
Skinner MA, Buller RM, Damon IK, Lefkowitz EJ, McFadden G, McInnes CJ, Mercer AA, Moyer RW, Upton C (2012) Poxviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (eds) Virus taxonomy: ninth report of the International Committee on taxonomy of viruses. Elsevier Academic Press, New York, pp 291–309
Adams MJ, Carstens EB (2012) Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses. Arch Virol 157:1411–1422
Adams MJ, Lefkowitz EJ, King AMQ, Harrach B, Harrison RL, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Mushegian AR, Nibert M, Sabanadzovic S, Sanfaçon H, Siddell SG, Simmonds P, Varsani A, Zerbini FM, Gorbalenya AE, Davison AJ (2017) Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses. Arch Virol 162:2505–2538
Upton C, Slack S, Hunter AL, Ehlers A, Roper RL (2003) Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J Virol 77:590–600
Afonso CL, Delhon G, Tulman ER, Lu Z, Zsak A, Becerra VM, Zsak L, Kutish GF, Rock DL (2005) Genome of deerpox virus. J Virol 79:966–977
Kummuneje K, Krogsrud J (1979) Contagious ecthyma (orf) in reindeer (Rangifer tarandus tarandus). Vet Rec 105:60–61
Horner GW, Robinson AJ, Hunter R, Cox BT, Smith R (1987) Parapoxvirus infections in New Zealand farmed red deer (Cervus elaphus). N Z Vet J 35:41–45
Scagliarini A, Vaccari F, Turrini F, Bianchi A, Cordioli P, Lavazza A (2011) Parapoxvirus infections of red deer Italy. Emerg Infect Dis 17:684–687
Tikkanen MK, McInnes CJ, Mercer AA, Büttner M, Tuimala J, Hirvelä-Koski V, Neuvonen E, Huovilainen A (2004) Recent isolates of parapoxvirus of Finnish reindeer (Rangifer tarandus tarandus) are closely related to bovine pseudocowpox virus. J Gen Virol 85:1413–1418
Klein J, Tryland M (2005) Characterisation of parapoxviruses isolated from Norwegian semi-domesticated reindeer (Rangifer tarandus tarandus). J Virol 2:79
Lance WR, Charles HP, DeMartini J (1983) Experimental contagious ecthyma in mule deer white-tailed deer pronghorn and wapiti. J Wildl Dis 19:165–169
Zarnke RL, Dietrich RA, Neiland KA, Ranglack G (1983) Serologic and experimental investigation of contagious ecthyma in Alaska. J Wildl Dis 19:170–174
Roess AA, Galan A, Kitces E, Li Y, Zhao H, Paddock CD, Adem P, Goldsmith CS, Miller D, Reynolds MG, Zaki SR, Damon IK (2010) Novel Deer-associated parapoxvirus infection in deer hunters. N Engl J Med 363:2621–2627
Falk ES (1978) Parapoxvirus infections in reindeer and muskox associated with unusual human infections. B J Dermatol 99:647–654
Smith KJ, Skelton HG, James WD, Lupton GP (1991) Parapoxvirus infections acquired after exposure to wildlife. Arch Dermatol 127:79–82
Kuhl JT, Huerter CJ, Hashish H (2003) A case of human orf contracted from a deer. Cutis 71:288–290
Kitchen M, Müller H, Zobl A, Windisch A, Romani N, Huemer HP (2014) Orf virus infection in a hunter presumably transmitted by game in Western Austria. Acta Dermatovenereol 94:212–214
Barker IK, Mehren KG, Rapley WA, Gangon AN (1980) Keratoconjunctivitis and oral/cutaneous lesion associated with poxvirus infection in reindeer. In: Montali RJ, Migaki G (eds) The comparative pathology of zoo animals. Smithsonian Institution Press, Washington DC, pp 171–177
Williams ES, Becerra VM, Graham TJ, Owens MJ, Nunamaker CE (1985) Spontaneous poxviral dermatitis and keratoconjunctivitis in free-ranging mule deer (Odocoileus hemionus) in Wyoming. J Wildl Dis 21:430–433
Patton JF, Nordhausen RW, Woods LW, MacLachlan NJ (1996) Isolation of a poxvirus from a black-tailed deer (Odocoileus hemionus columbianus). J Wildl Dis 32:531–533
Junge RE, Duncan MC, Miller E, Gregg D, Kombert M (2003) Clinical presentation and antiviral therapy for poxvirus infection in pudu (Pudu puda). J Zoo Wildl Med 31:412–418
Moerdyk-Schauwecker M, Eide K, Bildfell R, Baker RJ, Black W, Graham D, Thompson K, Crawshaw G, Rohrmann GF, Jin L (2009) Characterization of cervidpoxvirus isolates from Oregon, California and eastern Canada. J Vet Diagn Investig 21:487–492
Baughman B, Zhang S, Jin L, Pace LW, Cooley J, Yan L, Zhang MZ (2011) Diagnosis of deerpox virus infection in a white-tailed deer (Odocoileus virginianus) fawn. J Vet Diagn Investig 23:965–970
Bracht AJ, Armién AG, Carrillo C, O’Hearn ES, Fabian AW, Moran KE, Lu Z, Ariyakumer DS, Rasmussen JM, Metwally SA (2013) Isolation and characterization of a cervidpoxvirus from a goitered gazelle (Gazella sugutturosa) from a zoological park in Minnesota. J Zoo Wildl Med 44:589–595
Bildfell RJ, Thompson KA, Moerdyk-Schauwecker M (2010) Experimental deerpox infection in black-tailed deer (Odocoileus hemionus columbianus). J Wildl Dis 46:33–45
Afonso PP, Silva PM, Schnellrath LC, Jesus DM, Hu J, Yang Y, Renne R, Attias M, Condit RC, Moussatché N, Damaso CR (2012) Biological characterization and next-generation genome sequencing of the unclassified Cotia virus SPAn232 (Poxviridae). J Virol 86:5039–5054
Haller SL, Peng C, McFadden G, Rothenburg S (2014) Poxviruses and the evolution of host range and virulence. Infect Genet Evol 21:15–40
Inoshima Y, Morooka A, Sentsui H (2000) Detection and diagnosis of parapoxvirus by the polymerase chain reaction. J Virol Methods 84:201–208
VanDevanter DR, Warrener P, Bennett L, Schultz ER, Coulter S, Garber RL, Rose TM (1996) Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol 34:1666–1671
Li Y, Meyer H, Zhao H, Damon IK (2010) GC content-based pan-pox universal PCR assays for poxvirus detection. J Clin Microbiol 48:268–276
Wernike K, Hoffman H, Beer M (2015) Simultaneous detection of five notifiable viral diseases of cattle by single-tube multiplex real-time RT-PCR. J Virol Methods 217:28–35
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359
Milne I, Bayer M, Cardle L, Shaw P, Stephen G, Wright F, Marshall D (2010) Tablet-next generation sequence assembly visualization. Bioinformatics 26:401–402
Tcherepanov V, Ehlers A, Upton C (2006) Genome Annotation Transfer Utility (GATU): rapid annotation of viral genomes using a closely related reference genome. BMC Genom 7:150
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Jin L, McKay A, Green R, Xu L, Bildfell R (2013) Serosurvey for antibody to deerpox virus in five cervid species in Oregon USA. J Wildl Dis 49:186–189
Tu SL, Nakazawa Y, Gao J, Wilkins K, Gallardo-Romero N, Li Y, Emerson GL, Carroll DS, Upton C (2017) Characterization of Eptesipoxvirus a novel poxvirus from a microchiropteran bat. Virus Genes 53:856–867
Maclachlan NJ, Dubovi EJ, Barthold SW, Swayne DF, Winton JR (2017) Fenner’s veterinary virology, 5th edn. Elsevier Academic Press, New York
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the University of Florida, Institute of Food and Agricultural Science Cervidae Health Research Initiative, with funds provided by the State of Florida legislature.
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at University of Florida (IACUC Protocol #: 201609390).
Additional information
Handling Editor: YiMing Shao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sayler, K.A., Subramaniam, K., Jacob, J.M. et al. Characterization of mule deerpox virus in Florida white-tailed deer fawns expands the known host and geographic range of this emerging pathogen. Arch Virol 164, 51–61 (2019). https://doi.org/10.1007/s00705-018-3991-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3991-7