Abstract
Viral respiratory infections are raising serious concern globally. Asian medicinal plants could be useful in improving the current treatment strategies for influenza. The present study examines the activity of five plants from Bangladesh against influenza virus. MDCK cells infected with influenza virus A/Puerto Rico/8/34 (H1N1) were treated with increasing concentrations of ethyl acetate extracts, and their cytotoxicity (CC50), virus-inhibiting activity (IC50), and selectivity index (SI) were calculated. The ethyl acetate extract of fruits of Embelia ribes Burm. f. (Myrsinaceae) had the highest antiviral activity, with an IC50 of 0.2 µg/mL and a SI of 32. Its major constituent, embelin, was further isolated and tested against the same virus. Embelin demonstrated antiviral activity, with an IC50 of 0.3 µM and an SI of 10. Time-of-addition experiments revealed that embelin was most effective when added at early stages of the viral life cycle (0-1 h postinfection). Embelin was further evaluated against a panel of influenza viruses including influenza A and B viruses that were susceptible or resistant to rimantadine and oseltamivir. Among the viruses tested, avian influenza virus A/mallard/Pennsylvania/10218/84 (H5N2) was the most susceptible to embelin (SI = 31), while A/Aichi/2/68 (H3N2) virus was the most resistant (SI = 5). In silico molecular docking showed that the binding site for embelin is located in the receptor-binding domain of the viral hemagglutinin. The results of this study provide evidence that E. ribes can be used for development of a novel alternative anti-influenza plant-based agent.
Similar content being viewed by others
Introduction
Embelia ribes Burm. f. (Myrsinaceae), first described by Nicolaas Laurens Burman in 1768, is a woody climber distributed in the primary lowland and mountain forests of Bangladesh, Burma, Cambodia, India, Laos, peninsular Malaysia, Thailand, and Vietnam [1]. Ayurvedic medicine prescribes the dried fruits or “vidanga” to induce vomiting, to treat diabetic ulcers, to promote longevity, to abort, to expel intestinal worms, and to treat bronchitis [2]. As a vestige of Ayurveda in Cambodia, Laos and Vietnam, the dried fruits of E. ribes are taken internally to treat helminthiasis [3]. Asians settling in Britain used E. ribes to treat tapeworm, chest infection and skin ailments [4]. The dried fruits in the British Pharmaceutical Codex of 1934 were listed as an anthelmintic remedy to be taken at a dose of 4 g to 16 g daily. There is evidence that E. ribes is cardio-protective [5]. The fruits contain a series of red-colored alkyl-benzoquinones, including embelin, first isolated and characterized in 1941 [6], embelinol, and embeliol [7]. Embelin is an antibacterial [8] and anti-inflammatory [9] compound.
E. ribes was reportedly used by natives in India for the treatment of influenza in 1919 [10]. Influenza is one of the most dangerous viral respiratory infections of humans. The pandemic influenza A/H1N1 (Spanish flu) virus in 1918-19 claimed about 40 million lives, the Asian flu virus A(H2N2) in 1957 led to the deaths of almost 4 million people, and 2 million people died from the Hong Kong influenza (H3N2) virus in 1968 [11]. In 2009, WHO reported the spread of a novel pandemic influenza A (H1N1) virus in the US and Mexico (“swine flu”). The virus spread rapidly and caused 209,000 cases of infection and over 3205 deaths in 168 countries [12]. Vaccination can only provide a limited control of the spread of infection due to continuous antigenic drift [13]. For this reason, the search for inhibitors of influenza A virus among natural products is a promising route of selection of lead compounds for preclinical studies [14]. The adamantane derivatives amantadine, and rimantadine were identified in the 1960s as anti-influenza A drugs and were used for several decades for prophylaxis and treatment of influenza [15]. Oseltamivir (Tamiflu®), derived from shikimic acid from the fruits of Illicium verum Hook. f. (Illiciaceae) is a neuraminidase inhibitor that has been approved by the FDA [16]. However, the rapid emergence of viruses that are resistant to both neuraminidase inhibitors and adamantane derivatives has led to the need for the development of other inhibitors [17]. In this context, we examined the antiviral properties of five medicinal plants from Bangladesh, including E. ribes, against influenza virus A/Puerto Rico/8/34 (H1N1) in vitro. The aims of our study were: (i) to examine the antiviral properties of ethyl acetate extracts of five medicinal plants from Bangladesh against influenza virus in vitro, (ii) to identify at least one extract with strong antiviral potential (iii), to determine the range of virus-suppressing action of the major constituent in the most active extract against a panel of influenza viruses, and (iv) to determine the mode of anti-influenza activity of the major constituent. The ultimate goal of our study is to contribute to the development of safe, effective, and inexpensive antiviral plant-based material to treat influenza.
Materials and methods
Plant material and extraction procedure
Curcuma aeruginosa Roxb. (Zingiberaceae) (rhizomes) (Voucher no. SH123), E. ribes (fruits) (Voucher No. SH124), Ocimum basilicum L. (Lamicaeae) (seeds) (Voucher No. SH125), Terminalia bellirica (Gaertn.) Roxb. (Combretaceae) (fruits) (Voucher No. SH126), and Curcuma rubescens Roxb. (Zingiberaceae) (rhizomes) (Voucher No. SH127) were collected or purchased in Dhaka, Bangladesh, in January 2014 and identified by Professor Mohammed Rahmatullah, plant specialist at the Medicinal Plant Collection Wing, Faculty of Life Sciences, University of Development Alternative, Dhaka, Bangladesh. Samples were dried in an oven at 40 °C for 3 days and ground into a fine powder. Fifty grams of powder was extracted by cold maceration with 200 mL of ethyl acetate for 2 days. After filtration, liquid extracts were evaporated in vacuo and kept at -20 °C until testing.
High-performance liquid chromatography (HPLC) analysis
The HPLC method developed for embelin analysis was modified from a previous study [18]. The presence of embelin as a main constituent of the ethyl acetate extract of dried fruits of E. ribes was established by HPLC analysis, using embelin (CAS No. 550243; 98% purity; ChromaDex) as a reference. The quantification of embelin was performed using Agilent HPLC system (1290 Series) with UV-Vis detector. A very dilute solution of embelin analytical standard (CAS No. 550243; 98% purity; ChromaDex) was prepared in methanol and filtered through a 0.45-µm syringe filter, and 20 µL was injected into the HPLC system with an auto-injector attached to a reverse-phase column (Hypersil Gold C18) with 250 mm length and 4.6 mm diameter, which was maintained at 26 °C. Acetonitrile (analytical grade, RCI Labscan, Thailand) and purified water were chosen as mobile phases, with a ratio of 90:10. The flow rate was maintained at 0.75 mL/min, and the wavelength was set at 290 nm. The ethyl acetate extract was prepared in the same way as the standard and analyzed by HPLC.
Cells and viruses
The following influenza viruses, obtained from the collection of viruses of the Influenza Research Institute, St. Petersburg, Russia, were used in the study: A/Puerto Rico/8/34 (H1N1), A/Aichi/2/68 (H3N2), A/mallard/Pennsylvania/10218/84 (H5N2), A/California/07/09 (H1N1)pdm09, B/Malaysia/2506/04, and A/Vladivostok/02/09 (H1N1).
Prior to the experiment, the viruses were propagated in the allantoic cavities of 10-to-12 day-old chicken embryos for 48 h at 36 °C. The infectious titer of the virus was determined in MDCK cells (ATCC no. CCL-34) in 96-wells plates in alpha-MEM medium (Biolot, St. Petersburg, Russia).
Cytotoxicity assay
The microtetrazolium test (MTT) was used to study the cytotoxicity of the extracts and embelin [19]. Briefly, the extracts and compound were serially diluted threefold (from 300 to 4 µg/mL, with additional lower dilutions for separate experiments, if needed) in minimal essential medium (MEM). MDCK cells were incubated for 48 h at 37 °C in 5% CO2 in the presence of the dissolved substances. The degree of destruction of the cell monolayer was then determined by MTT. The cells were washed twice with saline, and a solution of 3-(4,5-dimethylthiazolyl-2) 2,5-diphenyltetrazolium bromide (ICN Biochemicals Inc., Ohio, USA) (0.5 mg/mL) in MEM was added to the wells. After a 1-h incubation, the wells were washed for 5 min with saline, and the formazan precipitate was dissolved in DMSO (0.1 mL per well). The optical density of cells was then measured on a Victor2 1440 multifunctional reader (Perkin Elmer, Finland) at a wavelength of 535 nm and plotted against the concentration of the extracts. Each concentration was tested in three replicates. The optical density was plotted against the concentration, and the 50% cytotoxic concentration (CC50) was calculated from the data obtained.
Antiviral assay
The extracts or embelin at increasing concentrations (4-300 µg/mL) were dissolved in MEM with trypsin (1 µg/mL) and incubated with MDCK cells at 36 °C for 1 h. The cell culture was then infected with the corresponding viruses at a multiplicity of infection (moi) of 0.01 and incubated for 1 h at 36 °C in the presence of 5% CO2. The culture medium was then removed and replaced with fresh medium containing the same concentrations of material to be tested. Plates were incubated at 36 °C in the presence of 5% CO2 for 24 h, followed by virus titer determination by TCID50 for 48 h. Each extract or embelin concentration was tested in quadruplicate. The 50% inhibitory concentration (IC50) and SI (CC50-to-IC50 ratio) were calculated from the data obtained. Oseltamivir carboxylate (Hoffmann LaRoche, Basel, Switzerland) and rimantadine (Sigma-Aldrich, Missouri, USA) were used as reference antiviral drugs.
Time-of-addition experiments
To determine the stage of the viral life cycle that is affected by embelin, cells were seeded in 24-well plates and incubated with influenza virus A/Puerto Rico/8/34 (H1N1) (moi, 10) for 1 h at 4 °C. After washing for 5 min with 0.1 mL of cold MEM (+4 °C) per well to remove unabsorbed virions, plates were incubated for 8 h at 36 °C at 5% CO2. The starting point of this incubation was referred as to 0 h. Embelin (final concentration 1.5, µmol/L) was dissolved in MEM, and cells were treated with this solution for the following time periods: -2 to -1 hpi (before infection); -1 to 0 hpi (during absorption); 0 to 1, 1 to 2, 2 to 4, 4 to 6, and 6 to 8 hpi. The treatment from -2 to -8 hpi was used as a positive control. In each case, after incubation, embelin was removed and cells were washed for 5 min with MEM. After 8 h of growth, the infectious titer of the virus in the culture medium and cells was determined as described above.
In a separate set of experiments, the effect of embelin on the cell-free virus was assessed. For this purpose, 0.5 mL of virus-containing culture medium (105 TCID50/mL) was mixed with embelin at a final concentration of 1.3 or 0.4 µM and incubated for 1 h at 36 °C at 5% CO2, following by virus titer determination by the TCID50 method as described above.
Hemagglutination inhibition assay
In order to assess the ability of Embelia ribes extract and embelin to interfere directly with HA receptor binding, we performed a hemagglutination inhibition test. Twofold dilutions of culture medium containing influenza A/Puerto Rico/8/34 virus (1:8 to 1:256) were mixed with Embelia ribes extract at final concentration 3 µg/mL or embelin (1.3 µM) and incubated for 1 hour at 36 °C at 5% CO2, after which an equal volume of 1% chicken erythrocytes was added. After incubation for 1 hour at 20 °C, the results were checked visually. Anti-hemagglutinin activity was evaluated by the ability of specimens to prevent virus-driven hemagglutination.
Docking studies
Crystal structures of H5 hemagglutinin from H5N2 influenza virus bound to a sialic acid structure found on avian (1JSN) and human (5E30) receptors were downloaded from the Protein Data Bank [20]. Hemagglutinin was bound to sialic acid attached to galactose via α-2,3 linkages as found in avian receptors (1JSN) and to α-2, 6 linkages as found in human receptors (5E30). The structure of embelin (CID 3218) was obtained from PubChem [21]. Autodock Vina [22] was used to dock embelin to the avian and human receptors. The HA1 protein of the hemagglutinin was used as the target receptor because it contained the sialic acid binding site reported in the literature [23]. Sialic acid was used as a control for docking. Embelin and the two sialic acids were prepared using the AutoDock tools by adding essential hydrogen atoms, Kollman united atom type charges, and solvation parameters [24]. Autogrid was used to generate the grid maps of 40 Å and 0.375 Å spacing around the binding site, which was selected using the sialic acid position bound to the H5 hemagglutinin in the crystal structures Nine docked positions were generated, and the position with the lowest binding energy (high negative) value was selected for analysis. Pymol [25] was used to visualise the docked structures, and Biovia Discovery Studio visualizer was used to plot the two-dimensional interaction figures [26].
Statistical analysis
All of the experiments were done in triplicate and analyzed using Graph Pad Prism v7 (Graph Pad software, San Diego, California). The values of viral titers were compared by Mann-Whitney test. The significance of the relationship between experimental parameters was assessed using linear regression (p < 0.05).
Results
The ethyl acetate extracts of five medicinal plants in Bangladesh were initially screened for their cytotoxicity in vitro to MDCK cells using a microtetrazolium test (MTT) (Table 1). Among the plants tested, E. ribes, C. rubescens, T. bellirica, and C. aeruginosa, with CC50 values of 6.3, 16.6, 20.8 and 23.9 µg/mL, respectively, were mildly toxic according to American National Cancer Institute guidelines [26]. An extract of O. basilicum did not demonstrate cytotoxicity up to the highest concentration used (300 µg/mL). To examine the antiviral effects of these extracts, we tested their ability to suppress the growth of influenza virus A/Puerto Rico/8/34 (H1N1) in MDCK cells (Table 1). Among the extracts tested, E. ribes demonstrated the highest level of virus-inhibiting activity (IC50 = 0.2 µg/mL), with an SI value of 32, supporting the notion that E. ribes contains one or more compounds that selectively suppresses the reproduction of influenza virus. C. aeruginosa, although possessing only moderate activity (SIs lower than 10, namely 6) could be considered a prospect as well. Since all of the specimens tested were unfractionated extracts, it cannot be ruled out that the active compound(s) contained in these mixtures might have much lower CC50 values.
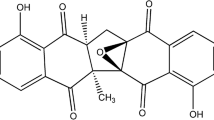
For further experiments, the most active extract (E. ribes) was used. There is evidence to suggest that benzoquinones possess antiviral activity [27,28,29], and we raised the question of whether the virus-inhibiting properties of extract of E. ribes were due to benzoquinones, in particular, its major constituent, embelin (Fig. 1). The presence of embelin in the ethyl acetate extract of dried fruits of E. ribes was confirmed by HPLC analysis. Both the embelin standard and the major peak in the extract demonstrated a sharp and massive peak at a retention time of 5.139 min in the HPLC profile (Fig. 2). A calibration curve of the standard was made by serial dilution from a concentration of 100 to 6.25 µg/mL. Excellent linearity was obtained, with a regression coefficient (r2) of 0.9994 and the regression equation y = 126.21 x + 162.57 (x = embelin concentration and y = average peak area. The amount of embelin present in the ethyl acetate extract was quantified as 60.7% (w/w). Embelin was tested for cytotoxicity and its ability to inhibit influenza virus A/Puerto Rico/8/34 (H1N1). The CC50 of embelin was found to be 0.9 µg/mL (3.1 µM), and its IC50 against the virus was 0.3 µM (SI = 10) (Table 2).
To further characterize the range of virus-suppressing action of embelin, we tested its activity against influenza viruses differing in their type and subtype (A(H1N1), A(H3N2), A(H5N2) and B), origin (human and avian) and susceptibility/resistance to currently available antivirals (e.g., rimantadine and oseltamivir). The results are summarized in Table 2. The viruses used in this study demonstrated different levels of susceptibility to embelin. The A/Puerto Rico/8/34 virus that was used for the screening was moderately sensitive, while the avian virus A/mallard/Pennsylvania/10218/84(H5N2) was the most susceptible. No correlation was observed between sensitivity to rimantadine and embelin or between oseltamivir and embelin.
To investigate the mode of anti-influenza activity of embelin, we performed time-of-addition experiments. Since different viral proteins play critical roles at different stages of the viral life cycle, results of such experiments could give an idea about which viral proteins are targeted by the compound. Both the whole extract of Embelia ribes and embelin were most active when applied at early stages of the viral life cycle (0-1 h postinfection [hpi]) (Fig. 3). At other time points, their inhibitory activity was lower. This suggests that embelin prevents virion absorption to the cell surface, either by blocking the cell receptor (-1 to 0 hpi) or, more effectively, by blocking the receptor-binding site of viral hemagglutinin (0 to1 hpi). Also, the fusion of the viral envelope with the endosome membrane, which occurs at an early stage of the viral life cycle, might also be affected by embelin. To test whether embelin is able to block virus replication by direct inactivation of extracellular virions, we incubated the cell-free virus with 1.3 µM of embelin and then determined the activity of the virus in cell culture. The infectious titer of the virus without embelin was 4.3 ± 0.5 TCID50/0.1 mL, and after incubation, with embelin it decreased to 3.8 ± 0.5 TCID50/0.1 mL. In addition, we performed a hemagglutination inhibition test to assess the ability of embelin and whole extract of Embelia ribes to inhibit binding of hemagglutinin to its receptor. The hemagglutination titer of the control virus was 1:128, while after incubation with E. ribes extract or embelin, the titer was four times lower (1:32). This suggests that both the whole extract and pure embelin prevent virus-induced agglutination of erythrocytes (Fig. 4).
Time-of-addition experiment using 3 µg of Embelia ribes extract per mL (A) or 1.3 µM embelin (B) against influenza virus A/Puerto Rico/8/34 (H1N1). Embelin was added and removed at the time points indicated on the x-axis. Pre-cooled cell culture was infected with the virus and incubated at +4 °C for 1 h. The unbound virus was removed, and cells were kept at +36 °C. The moment of transfer corresponds to time point zero
Inhibition of influenza-virus-induced hemagglutination by Embelia ribes extract (columns 1 and 2) and pure embelin (columns 3 and 4) compared to virus control (saline instead of compounds, lines 5 and 6). Twofold dilutions of influenza virus A/Puerto Rico/8/34 (H1N1) (indicated on the right) were incubated with E. ribes extract (3 µg/mL) or embelin (1.3 µM) in the wells of round-bottom plates, and 1% chicken erythrocytes were added. The plates were incubated for 1 h at 20 °C for sedimentation of erythrocytes
To confirm that embelin interacts directly with the hemagglutinin, in silico molecular modeling of this interaction was performed. The entry process of influenza virus is well understood and has been reported in detail in the literature. Several studies have shown the development and use of hemagglutinin inhibitors to be an effective method of controlling the spread of influenza virus [23]. Hemagglutinin is important because it recognizes α-2,3- or α-2,6 linked terminal sialic acids (SAs) in membrane glycoprotein receptors on the host cell, and therefore can mediate the virus-cell adsorption and endocytosis for virus entry. Hence, inhibitors targeting hemagglutinin are extremely useful [24]. The docking results provided evidence that embelin was able to dock to the same binding pocket as sialic acid. The binding energy of the best-docked position for the α-2,3 SA binding pocket as found on avian receptors was -5.3 kcal/mol. Figure 5A shows the docked position of embelin within the hemagglutinin of influenza virus. For comparison, the docked position of α-2,3 sialic acid is shown in Fig. 6A. Compared with embelin, α-2,3 sialic acid showed more stable binding with a binding free energy value of -6.6 kcal/mol. The residues within 4 Å of the two ligands are shown in Fig. 7A. Embelin formed a hydrogen bond with Tyr91, whereas sialic acid is seen to form hydrogen bonds with Tyr91, Val131, His179, and Gln222. Common residues within 4 Å of both ligands are Trp149, Glu186, Gln222, Gly224, and Pro181.
Two-dimensional plot of interactions of embelin with avian H5 hemagglutinin. Tyr91 forms a hydrogen bond with the OH group of C14 of embelin (A). Two-dimensional plot of interactions of a sugar molecule with avian H5 hemagglutinin. α-2-3 sialic acid forms extensive hydrogen bonds with pocket residues
Figures 5B and 6B show the docked positions of embelin and α-2,6 sialic acid, respectively. The binding free energy for embelin was –5.2 kcal/mol as compared with -6.2 kcal/mol when sialic acid was docked in the HA binding pocket. The two-dimensional plot of interactions of the docked structures shown in Fig. 8 shows that sialic acid formed hydrogen bonds with Tyr98, Val135, Ser136, Ser137, Glu190. Of these residues, embelin was able to interact with Glu190 only, while other residues with which embelin formed hydrogen bonds were Arg193, Ser227, and Gly228. Tyr98 and Val135 were found in the 4-Å region around embelin but not directly interacting with it.
Two-dimensional plot of interactions of embelin with human H5 hemagglutinin. Several hydrogen bonds, pi bonding and pi-alkyl bonds are responsible for the stable binding of embelin to the α-2-6 sialic acid binding pocket (A).Two-dimensional plot of interactions of a sugar molecule with human H5 hemagglutinin. The α-2-6 sialic acid forms extensive hydrogen bonds with pocket residues (B)
Discussion
We provide evidence that embelin, which is the main constituent of E. ribes, a plant that was used to treat influenza almost one hundred years ago, inhibits replication of influenza viruses. Besides substantiating a traditional Ayurvedic claim, our results provide support for the contention that embelin could be used as a starting molecular scaffold for the design of new antiviral agents.
Embelin is the major constituent of the extract, so with high probability, the main properties of the extract are due to embelin. Despite this, the activity of the whole extract was higher than that of purified embelin. A similar phenomenon has been described recently [30], and in both cases, it can be suggested that minor components of the extract could contribute antiviral activity, probably affecting different targets. We cannot rule out that the combination of embelin and minor fractions of the extract, either cell- or virus-targeted, can have an additive effect, thus enhancing the activity of embelin. Due to the low amounts of minor fractions in the extract and the small amount of the extract itself, we were unable to identify these minor constituents. Further studies are therefore needed to determine the exact mechanism of the virus-inhibiting action of the E. ribes extract.
Extracts of fruits of E. ribes have been used to treat various disorders, suggesting that the inhibitory effects of embelin are not specific for an individual pathogen or process. There is evidence demonstrating that embelin has the ability to interact in mammalian cells with several proteins, such as the X-linked inhibitor of apoptosis protein [9, 31], signal transducer and activator of transcription 3 [32], peroxisome proliferator-activated receptor-γ [33], and plasminogen activator inhibitor-1 [34]. Embelin has also been predicted in in silico molecular docking studies to bind to collagen and human neutrophil elastase [35]. Thus, in addition to the direct virus-inhibiting activity we have demonstrated, we cannot rule out the possibility that embelin could also inhibit the function(s) of cellular factors involved in virus replication. Embelin has an SI of 5 to 31, depending on the virus used, suggesting that the safe dose range of this compound as a therapeutic agent is quite narrow. According to Omura [36], the SI of antiviral agents should be more than 100 to indicate a useful effect on viral inhibition in animal experiments. However, a body of experimental evidence already exists that shows that embelin is not toxic when given orally to rodents. Daily oral administration of embelin (25 and 50 mg/kg/day) for three weeks did not cause side effects in rats [37]. In another study, embelin was subjected to a toxicity evaluation that included subacute, chronic, and reproductive toxicity testing and teratological investigations in laboratory animals (e.g., mice, rats, and monkeys). The results did not indicate adverse effects, suggesting that embelin is a safe compound [38]. At this time, we cannot explain this discrepancy. Chiang et al. tested an aqueous extract of O. basilicum against adenovirus and found an SI of 8.4 [39]. However, O. basilicum, or basil, is used in food and is not toxic to humans [40]. In the same study, the antiviral drug zalcitabine (2′-3′-dideoxycytidine) exhibited an SI of 10.1 [39]. The concept of SI in natural products antiviral research could therefore be revised.
The SI of embelin was considerably lower than that of the ethyl acetate extract of the dried fruit of E. ribes against influenza virus A/Puerto Rico/8/34 (H1N1), implying the presence of other active components. Embelin was tested against five other influenza viruses and demonstrated the highest activity against avian influenza virus A/mallard/Pennsylvania/10218/84 (H5N2), with an IC50 equivalent to that of oseltamivir and an SI of 31. The docking experiment also provides evidence that embelin binds to the α-2,3 binding pocket of hemagglutinin. At present, it is not clear whether all H5 viruses, or more generally, avian influenza viruses, are especially sensitive to embelin. Therefore, in future studies, the panel of viruses tested should be expanded. Despite embelin having demonstrated some effect on the infectivity of the virus when it is incubated with extracellular virions, these differences were not statistically significant. This suggests that the binding of embelin to HA might be comparatively weak and that specific modifications of its chemical structure could improve its potency. On another hand, despite the virus-inhibiting activity of embelin at the stage of virus absorption (-1 to 0 hpi), the highest level of activity was observed at the stage of endosome formation and membrane fusion (0 to 1 hpi). This could suggest that in addition to the SA-binding pocket of hemagglutinin, embelin has an additional viral or cellular target that is involved in viral reproduction at early stages of the cycle. The results of the hemagglutination inhibition assay demonstrate that whole extract and pure hemagglutinin both suppress the process of influenza-virus-induced agglutination of erythrocytes to an approximately equal extent. This also suggests that interaction of either embelin or other components in the extract with other (non-hemagglutinin) targets contributes to the total virus-inhibiting activity.
In conclusion, we have demonstrated that an ethyl acetate extract of dried fruits of E. ribes possesses antiviral activity against influenza virus A/Puerto Rico/8/34 (H1N1), confirming that this plant can be used to treat influenza. Its major constituent, embelin, suppressed virus replication in cell culture at early stages of the viral life cycle (0 to 1 hpi) and demonstrated inhibitory activity when applied directly to extracellular virions. In a computer simulation, embelin was predicted to interact with the receptor-binding site of viral hemagglutinin. Both E. ribes extract and embelin are therefore beneficial to human health and have the potential to be used for medical purposes for the treatment of influenza. To the best our knowledge, this is the first attempt to assess the virus-inhibiting properties of E. ribes and to identify the active antiviral compounds in the extract. Further efforts in this direction would focus on optimizing the basic structure of this compound to develop novel effective antivirals that currently have no analogs in current clinical practice.
References
Dubearnes A, Julius A, Utteridge TMA (2015) A synopsis of the genus Embelia in Peninsular Malaysia and Singapore Studies in Malaysia Myrsinaceae III. Kew Bull 70:2
Murthy S, Samhita S (2012) Chaukhamba Orientala, Varansi
Perry LM, Metzger J (1980) Medicinal plants of East and Southeast Asia: attributed properties and uses. The MIT Press, Cambridge
Aslam M (1996) Asian medicine and its practice in Britain. In Trease and Evan’s pharmacognosy, 14th edn. WB Saunders Company Ltd., London
Bhandari U, Ansari MN, Islam F (2008) Cardioprotective effect of aqueous extract of Embelia ribes Burm fruits against isoproterenol-induced myocardial infarction in albino rats. Indian J Exp Biol 46:35–40
Chopra RN, Nayar SL, Chopra IC (1996) Glossary of Indian medicinal plants. NISCOM, New Delhi
Haq K, Ali M, Siddiqui AW (2005) New compounds from the seeds of Embelia ribes. Pharmazie 60:69–71
Chitra M, Devi CS, Sukumar E (2003) Antibacterial activity of embelin. Fitoterapia 74:401–403
Ahn KS, Sethi G, Aggarwal BB (2007) Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol 71:209–219
Menon K (1919) Embelia ribes—a medicine for influenza. Indian For 45:210
Swartz KA, Luby JP (2007) Pandemic influenza a primer. Tex Med 103:31–34
Saxena SK, Mishra N, Saxena R, Saxena S (2009) Swine flu: influenza A/H1N1 2009: the unseen and unsaid. Future Microbiol 4:945–947
Cox NM (2005) Pandemic influenza: overview of vaccines and antiviral drugs. Yale J Biol Med 78:321–338
Arakawa T, Yamasaki H, Ikeda K, Ejima D, Naito T, Koyama AH (2009) Antiviral and virucidal activities of natural product. Curr Med Chem 16:2485–2497
Scholtissek C, Quack G, Klenk HD, Webster RG (1998) How to overcome resistance of influenza A viruses against adamantane derivatives. Antivir Res 37:83–95
Saladino R, Barontini M, Nencioni M, Sgarbanti R, Palamara AT (2010) Current advances in anti-influenza therapy. Curr Med Chem 17:2101–2140
Dixit R, Khandaker G, Ilgoutz S, Rashid H, Booy R (2013) Emergence of oseltamivir resistance: control and management of Influenza before, during and after the pandemic. Infect Disord Drug Targets 13:34–45
Kulkarni SV, Damle MC (2015) Development and validation of Stability Indicating HPLC method for estimation of Embelin in Embelia tsjeriam cottam (Vidanga). Int J Ayurvedic Med 6:243–249
Mosmann T (1980) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242
Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA, Wang J, Yu B, Zhang J, Bryant SH (2016) PubChem substance and compound databases. Nucleic Acids Res 44:D1202–D1213
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461
Das K, Aramini JM, Ma LC, Krug RM, Arnold E (2010) Structures of influenza A proteins and insights into antiviral drug targets. Nat Struct Mol Biol 17:530–538
Yang J, Li M, Shen X, Liu S (2013) Influenza A virus entry inhibitors targeting the hemagglutinin. Viruses 5:352–373
DeLano WL (2002) Pymol: an open-source molecular graphics tool. CCP4 Newsl Protein Crystallogr 40:82–92
Biovia DS (2015) Discovery studio modeling environment, release, 4. Dassault Systemesc, San Diego
Hashim NM, Rahmani M, Ee GCL, Sukari MA, Yahayu M, Oktima W, Ali AM, Go R (2012) Antiproliferative activity of xanthones isolated from Artocarpus obtusus. J Biomed Biotechnol 130627:1–9
Ono K, Nakane H, Shimizu S, Koshimura S (1991) Inhibition of HIV-reverse transcriptase activity by asterriquinone and its analogues. Biochem Biophys Res Commun 174:56–62
Bogdanova NS, Pershin GN, Nikolaeva IS, Grinev AN, Shvedov VI (1970) Antiviral activity of p-benzoquinone and hydroquinone derivatives. Farmakol Toksikol 33:488–496
Wagner RR (1951) Studies on the inactivation of influenza virus; comparison of the effects of p-benzoquinone and various inorganic oxidizing agents. Yale J Biol Med 23:288–298
Astani A, Reichling J, Schnitzler P (2011) Screening for antiviral activities of isolated compounds from essential oils. Evid Based Complement Altern Med 2011:253643. https://doi.org/10.1093/ecam/nep187
Nikolovska-Coleska Z, Xu L, Hu Z, Tomita Y, Li P, Roller PP, Wang R, Fang X, Guo R, Zhang M, Lippman ME (2004) Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem 47:2430–2440
Heo JY, Kim HJ, Kim SM, Park KR, Park SY, Kim SW, Nam D, Jang HJ, Lee SG, Ahn KS, Kim SH (2011) Embelin suppresses STAT3 signalling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase PTEN. Cancer Lett 308:71–80
Dai Y, Qiao L, Chan KW, Yang M, Ye J, Ma J, Zou B, Gu Q, Wang J, Pang R, Lan HY (2009) Peroxisome proliferator-activated receptor-γ contributes to the inhibitory effects of embelin on colon carcinogenesis. Cancer Res 69:4776–4783
Chen F, Zhang G, Hong Z, Lin Z, Lei M, Huang M, Hu L (2014) Design, synthesis, and SAR of embelin analogues as the inhibitors of PAI-1 (plasminogen activator inhibitor-1). Bioorg Med Chem Lett 24:2379–2382
Radhakrishnan N, Gnanamani A (2014) 2, 5-dihydroxy-3-undecyl-1, 4-benzoquinone (Embelin)-A second solid gold of India—a review. Int J Pharm Pharmacol Sci 6:23–30
Omura S (ed) (1992) The search for bioactive compounds from microorganisms. Springer, New York. ISBN 978-1-4612-4412-7
Naik SR, Niture NT, Ansari AA, Shah PD (2013) Anti-diabetic activity of embelin: involvement of cellular inflammatory mediators, oxidative stress and other biomarkers. Phytomedicine 20:797–804
Johri RK, Dhar SK, Pahwa GS, Sharma SC, Kaul JL, Zutshi U (1990) Toxicity studies with potassium embelate, a new analgesic compound. Indian J Exp Biol 28:213–217
Chiang LC, Ng LT, Cheng PW, Chiang W, Lin CC (2005) Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol 32:811–816
Acknowledgements
We would like to thank Mr. Rajesh Sreedharan Nair for assisting with HPLC work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest. This research was supported by a Grant from the Malaysian Ministry of Education (FRGS/1/2014/SG01/UNIM/02/1). We acknowledge the support.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals.
Informed consent
This article does not contain any studies with human participants.
Additional information
Handling Editor: Zhongjie Shi.
Rights and permissions
About this article
Cite this article
Hossan, M.S., Fatima, A., Rahmatullah, M. et al. Antiviral activity of Embelia ribes Burm. f. against influenza virus in vitro. Arch Virol 163, 2121–2131 (2018). https://doi.org/10.1007/s00705-018-3842-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3842-6